Why medicines have crazy names?
Medicines are known by their name just as humans are known by their names. Pharmaceutical companies have to name them to identify individually because a company can not sell their products without naming them. The brand name is the important factor of a product after the product's quality and packaging from the consumer's points
of view.
A brand is a name, logo, design or a symbol which allows us to differentiate one product from another one.
A drug which is being marketed by a pharmaceutical company has three names: Chemical name, Generic name, Brand name.
Chemical Name: It is decided on the basis of the chemical structure of the drug mainly used by the researchers. Its chemical name is very difficult to pronounce and remember because it is
long.
Generic Name: Drug’s official name is generic name throughout its lifetime. The generic name is mainly used by professionals in the medical field and is usually created when a new drug is ready to be marketed.
Brand Name: Although the generic name is in the public domain, the brand name is owned by the pharmaceutical products manufacturer and can be created as soon as the generic name has been approved.
Government Notification on generic and brand name implementation:
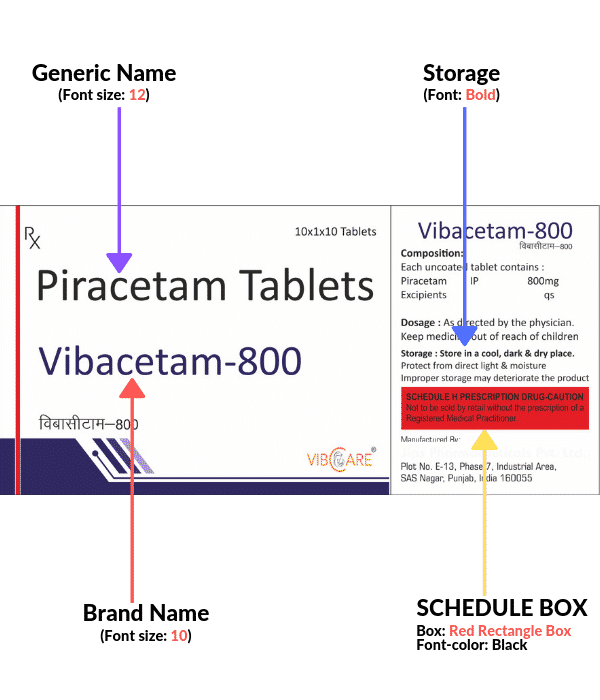
It is mandatory for all the pharma companies to print generic names of the drug larger than the brand name.
It is suggested to print generic name two font larger than brand name font size, said the Union health ministry.
The change in font size is mandatory for all type of formulations, except combinations of vitamins
and FDC (fixed-dose combinations) consisting of more than 3 molecules.
1. For products with Brand Name and generic name
- Fonts for the Generic name should be 2 points bigger than the font used for the Brand name. For Eg. Brand Name: 10 points
Generic Name: 12 points - The Type of font for Generic and Brand Name should be the same.
- Italic, Underline for fonts of Generic name /Brand name should not be used.
- The generic name for the carton/mono cartons /: Can be 70 % of Black Or in any other color which is used for the generic name in existing artwork.
- Generic Name on the foil: 100% black
- Text Matter should be in Calibri/Arial/Myriad Pro
- No changes in existing color schemes and carton designs.
- Text matter in existing artworks to be as per the guidelines.
- Complete red box for schedule H as per new guidelines
- Storage conditions in bold
- All the text of brand and generic to be in Upper Lower Case
For Eg. Brand Name: Acrimol
Generic name: Aceclofenac, Paracetamol Tablets
2. For products having 3 or more ingredients
- For products having 3 or more ingredients, Round Bracket will be put for the Brand name in the same color of the brand name.
- Complete red box for schedule H, H1 as per new guidelines
- Storage conditions in bold
- Text matter in existing artworks to be as per the guidelines
- All designs to remain the same
3. For products with Generic name only
- Complete red box for schedule H, H1 as per new guidelines
- Storage conditions in bold
- Text matter in existing artworks to be as per the guidelines
- All the text of generic to be in Upper Lower Case
Go through Checklist for preparation of Artworks as per Govt. Notification on generic and brand name and schedule H implementation.