Govt. Notification on generic and brand name implementation

Vibcare Pharma
26/11/18
Checklist for preparation of Artworks as per Govt. Notification on generic and brand name and schedule H implementation
a) For products with Brand Name and generic name
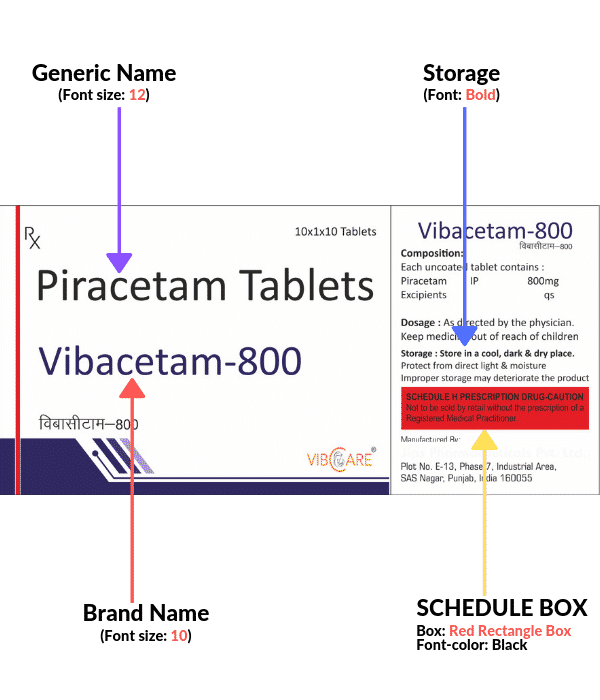
- Fonts for the Generic name should be 2 points bigger than the font used for the Brand name.
For Eg. Brand Name: 10 points
Generic Name: 12 points
- The Type of font for Generic and Brand Name should be the
- Italic, Underline for fonts of Generic name /Brand name should not be used.
- The generic name for the carton/mono cartons /: Can be 70 % of Black Or in any other color which is used for a generic name in existing
- Generic Name on the foil: 100% black
- Text Matter should be in Calibri/Arial/Myriad Pro
- No changes in existing color schemes and carton
- Text matter in existing artworks to be as per the guidelines.
- Complete red box for schedule H as per new guidelines
- Storage conditions in bold
- All the text of brand and generic to be in Upper Lower Case
For Eg. Brand Name: Vibcacetam
Generic name: Piracetam
b) For products having 3 or more ingredients
- For products having 3 or more ingredients, Round Bracket will be put for the Brand name in the same color of a brand name. NO other change in font size of Brand and generic is required.
- Complete red box for schedule H, H1 as per new guidelines
- Storage conditions in bold
- Text matter in existing artworks to be as per the guidelines
- All designs to remain same
c) For products with Generic name only
- Complete red box for schedule H, H1 as per new guidelines
- Storage conditions in bold
- Text matter in existing artworks to be as per the guidelines
- All the text of generic to be in Upper Lower Case
IMPORTANT:
1) Do not break the sentence in the schedule box.
2) Maintain the font size and font of this box as per the remaining text on artwork
| Existing | Proposed |
| If it contains a substance specified in Schedule G, be labeled with the words:
Caution: It is dangerous to take this preparation except under medical supervision
conspicuously printed and surrounded by a line within which there shall be no other words |
If it contains a drug substance specified in Schedule G, be labeled with following words in legible black colored font size in a completely Red rectangular box:
SCHEDULE G PRESCRIPTION DRUG-CAUTION |
| If it contains a substance specified in Schedule H, be labeled with the symbol Rx and conspicuously displayed on the left top comer of the label and be also labeled with the following words:
Schedule H Drug-Warning To be sold by retail on the prescription of a Registered Medical Practitioner only |
If it contains a drug substance specified in Schedule H, be labeled with symbol Rx and conspicuously displayed on the left top corner of the label and shall also be labeled with the following words in legible black colored font size in a completely Red rectangular box:
SCHEDULE H PRESCRIPTION DRUG-CAUTION
|
| If it contains a substance specified in Schedule H and comes within the purview of the [Narcotic Drugs and Psychotropic Substances Act, 1985 (61 of 1985)]2 be labeled with the symbol NRx which shall be in red and conspicuously displayed on the left top comer of the label, and be also labelled with the following words:
Schedule H Drug-Warning To be sold by retail on the prescription of a Registered Medical Practitioner only |
If it contains a drug substance specified in Schedule H and comes within the purview of the Narcotic Drugs and Psychotropic Substances Act, 1985 (61 of 1985) be labeled with symbol NRx which shall be in red and conspicuously displayed on the left top corner of the label and shall also be labeled with the following words in legible black coloured font size in completely Red rectangular box:
SCHEDULE H PRESCRIPTION DRUG-WARNING
|
| If it contains a substance specified in Schedule X, be labeled with the symbol XRx which shall be in red conspicuously displayed on the left top comer of the label, and be also labeled with the following words:
Schedule X Drug-Warning To be sold by retail on the prescription of a Registered Medical Practitioner only |
If it contains a drug substance specified in Schedule X, be labeled with symbol XRx which shall be in red and conspicuously displayed on the left top corner of the label and shall also be labeled with the following words in legible black colored font size in a completely Red rectangular box:
SCHEDULE X PRESCRIPTION DRUG-WARNING
|
| If it contains a substance specified in Schedule H1, the drug formulation shall be labeled with the symbol Rx which shall be in red and conspicuously displayed on the left top comer of the label, and shall also be labeled with the following words in a box with a red
Schedule H1 Drug-Warning: - It is dangerous to take this preparation except in accordance with the medical advice |
If it contains a drug substance specified in Schedule H1, be labeled with symbol Rx which shall be in red and conspicuously displayed on the left top corner of the label and shall also be labeled with the following words in legible black colored font size in a completely Red rectangular box:
SCHEDULE H1 PRESCRIPTION DRUG-CAUTION |
| If it contains a drug substance specified in
Schedule H1 and comes within the purview of the Narcotic Drugs and Psychotropic Substances Act, 1985 (61 of 1985) be labeled with symbol NRx which shall be in red and conspicuously displayed on the left top corner of the label and shall also be labeled with the following words in legible black coloured font size in completely Red rectangular box: SCHEDULE H1 PRESCRIPTION DRUG-CAUTION |

Copy Link