Government has banned 328 FDC
The Government has banned 328 FDC
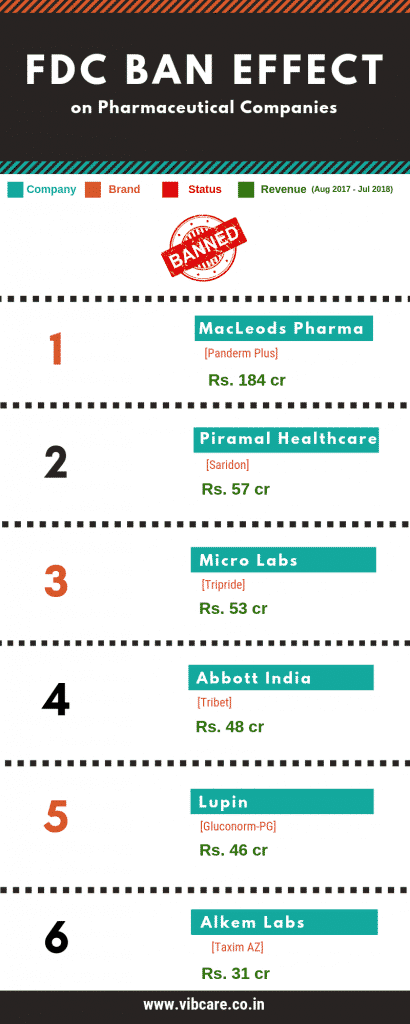
The Health Ministry has banned the manufacture, sale, and distribution of 328 Fixed Dose Combination (FDC) drugs with an immediate effect on September 12, 2018. The Indian pharmaceutical market size is estimated at around Rs 1.18-1.2 lakh crore, and FDCs reportedly make up approximately 50% of the sales. Here's what you need to know about them:
FDC means a combination of two or more drugs in a fixed dosage ratio. Some of the 328 banned FDC were: Cefixime + Azithromycin, Metronidazole + Norfloxacin, and Paracetamol + Propyphenazone + Caffeine (trade name Saridon), Ofloxacin + Ornidazole Suspension.
Why FDCs are so popular?
The simple answer to this question is cost. It is very cheap to buy Fixed dosage combination. Instead of buying two or more different medicines, a patient can buy only one FDC medicine to treat multiple disease symptoms. Pharmaceutical companies love them because it is far cheaper and quicker to combine existing active ingredients to manufacture new products than to discover new medicines and manufacture them separately.
Why has the government banned them?
The central government took this decision after the Drugs Technical Advisory Board recommended that "there is no therapeutic justification" for the ingredients contained in the banned 328 FDC drugs and that these medicines may involve risk to the patient.
Also, All India Drug Action Network (AIDAN), a network of NGOs that work to increase accessibility and improve the usage of essential medicines, said the people have been supplied with non-standard medicines for long.
How was Vibcare Pharma affected?
None of our products were affected by this ban on FDC. Do check our complete products list.
Here is a complete list of the banned drugs:
- Aceclofenac (SR) + Paracetamol
- Aceclofenac + Paracetamol + Famotidine
- Aceclofenac + Paracetamol + Rabeprazole
- Aceclofenac + Zinc Carnosine
- Acetaminophen + Guaifenesin + Dextromethorphan + Chlorpheniramine
- Acetaminophen + Loratadine + Ambroxol + Phenylephrine
- Acriflavine + Thymol + Cetrimide
- Acrivastine + Paracetamol + Caffeine + Phenylephrine
- Albuterol + Bromhexine + Theophylline
- Albuterol + Etofylline + Bromhexine + Menthol
- Alginic Acid + Sodium Bicarbonate + Dried Aluminium Hydroxide + Magnesium Hydroxide
- Allantoin + Dimethieone + Urea + Propylene + Glycerin + Liquid Paraffin
- Ambroxol + Levocetirizine + Phenylephrine + Guaiphenesin + Menthol
- Ambroxol + Guaifenesin + Phenylephrine + Chlorpheniramine
- Ambroxol + Salbutamol + Choline Theophyllinate + Menthol
- Ambroxol + Salbutamol + Theophylline
- Ambroxol + Terbutaline + Dextromethorphan
- Ambroxol+ Guaiphenesin + Ammonium Chloride + Phenylephrine + Chlorpheniramine Maleate + Menthol
- Ammonium Chloride + Dextromethorphan + Cetirizine + Menthol
- Ammonium Chloride + Sodium Citrate + Chlorpheniramine Maleate + Menthol
- Ammonium Citrate + Vitamin B 12 + Folic Acid + Zinc Sulphate
- Amoxicillin + Cefixime + Potassium Clavulanic Acid
- Amoxicillin + Dicloxacillin
- Amoxicillin 250 mg + Potassium Clavulanate Diluted 62.5
- Amoxycillin + Dicloxacillin + Serratiopeptidase
- Amoxycillin + Tinidazole
- Ascorbic Acid + Manadione Sodium Bisulphate + Rutin + Dibasic Calcium Phosphate + Adrenochrome mono Se
- Atorvastatin + Vitamin D3 + Folic Acid + Vitamin B12 + Pyridoxine
- Azithromycin + Acebrophylline
- Azithromycin + Ambroxol
- Azithromycin + Cefixime
- Azithromycin + Cefpodoxime
- Azithromycin + Levofloxacin
- Azithromycin + Ofloxacin
- Becloemthasone + Clotrimazole + Chloramphenicol + Gentamycin + Lignocaine Ear drops
- Beclomethasone + Clotimazole + Neomycin + lodochlorohydroxyquinone
- Beclomethasone + Clotrimazole + Gentamicin + lodochlorhydroxyquinoline
- Beclomethasone Diproprionate + Neomycin + Tolnaftate + lodochlorhydroxyquinoline +Chlorocresol
- Benfotiamine + Metformin
- Benzoxonium Chloride + Lidocaine
- Betahistine + Ginkgo Biloba Extract + Vinpocetine + Piracetam
- Betamethasone + Fusidic Acid + Gentamycin + Tolnaftate + lodochlorhydroxyquinoline (ICHQ)
- Betamethasone + Gentamicin + Tolnaftate + lodochlorhydroxyquinoline
- Betamethasone + Gentamycin + Zinc Sulphate + Clotrimoazole + Chlorocresol
- Betamethasone + Neomycin + Tolnaftate + lodochlorohydroxyquinoline + Cholorocresol
- Borax + Boric Acid + Naphazoline + Menthol + Camphor + Methyl Hydroxy Benzoate
- Bromhenxine + Phenylephrine + Chlorpheniramine + Paracetamol
- Bromhexine + Cetrizine + Phenylephrine IP+Guaifenesin + Menthol
- Bromhexine + Dextromethorphan
- Bromhexine + Dextromethorphan + Phenylephrine + Menthol
- Bromhexine + Phenylephrine + Chlorepheniramine Maleate
- Caffeine + Paracetamol + Chlorpheniramine
- Caffeine + Paracetamol + Phenylephrine + Cetirizine
- Calcium Gluconate + Chlorpheniramine + Vitamin C
- Calcium Gluconate + Levocetirizine
- Cefixime + Levofloxacin
- Cefixime + Linezolid
- Cefpodoxime Proxetil + Levofloxacin
- Cefuroxime + Linezolid
- Cephalexin + Neomycin + Prednisolone
- Certirizine + Phenylephrine + Paracetamol + Caffeine + Nimesulide
- Cetirizine + Acetaminophen + Dextromethorphan + Phenyephrine + Zinc Gluconate
- Cetirizine + Ambroxol + Guaiphenesin + Ammonium Chloride + Phenylephrine + Menthol
- Cetirizine + Dextromethorphan + Ambroxol
- Cetirizine + Dextromethorphan + Bromhexine + Guaifenesin
- Cetirizine + Dextromethorphan + Phenylephrine + Tulsi
- Cetirizine + Dextromethorphan + Phenylephrine + Zinc Gluconate + Paracetamol + Menthol
- Cetirizine + Dextromethorphan + Zinc Gluconate + Menthol
- Cetirizine + Diethyl Carbamazine
- Cetirizine + Phenylephrine + Dextromethorphan + Menthol
- Cetirizine + Phenylephrine + Paracetamol + Ambroxol + Caffeine
- Cetirizine + Phenylephrine + Paracetamol + Zinc Gluconate
- Cetririzine + Nimesulide + Phenylephrine
- Chlopheniramine Maleate + Dextromethorphan + Guaiphensin + Phenylephrine
- Chloramphenicol + Beclomethasone + Clomitrimazole + Lignocaine
- Chloramphennicol + Lignocaine + Betamethasone + Clotrimazole + Ofloxacin + Antipyrine
- Chlorphaniramine + Ammonium Chloride + Sodium Chloride
- Chlorpheniramine + Ammonium Chloride + Chloroform + Menthol
- Chlorpheniramine + Ammonium Chloride + Noscapine + Sodium Citrate
- Chlorpheniramine + Codeine + Sodium Citrate + Menthol Syrup
- Chlorpheniramine + Dextromethorphan + Phenylephrine + Paracetamol
- Chlorpheniramine + Paracetamol + Pseudoephedrine + Caffeine
- Chlorpheniramine + Phenylephrine + Caffeine
- Chlorpheniramine + Phenylephrine + Dextromethophan + Menthol
- Chlorpheniramine + Phenylephrine + Paracetamol + Zink Gluconate
- Chlorpheniramine + Terpin + Antimony Potassium Tartrate + Ammonium Chloride + SodiumCitrate + Menthol
- Chlorpheniramine + Vasaka + Tolubalsm + Ammonium Chloride + Sodium Citrate + Menthol
- Chlorpheniramine + Vitamin C
- Chlorpheniramine Maleate + Ammonium Chloride + Sodium Citrate
- Chlorpheniramine+Ammonium Chloride + Menthol
- Chlorpromazine + Trihexyphenidyl
- Chromium Polynicotinate + Metformin
- Cilnidipine + Metoprolol Succinate + Metoprolol Tartrate
- Ciprofloxacin + Fluocinolone + Clotrimazole + Neomycin + Chlorocresol
- Ciprofloxacin + Fluticasone + Clotrimazole + Neomycin
- Ciprofloxacin + Phenazopyridine
- Clidinium + Paracetamol + Dicyclomine + Activated Dimethicone
- Clindamycin + Clotrimazole + Lactic Acid Bacillus
- Clindamycin + Telmisartan
- Clobetasol + Gentamicin + Tolnaftate + lodochlorhydroxyquinone + Ketoconazole
- Clobetasol + Neomycin + Miconazole + Clotrimazole
- Clobetasol + Neomycin + Miconazole + Zinc Sulphate
- Clobetasol + Ofloxacin + Ketoconazol + Zinc Sulphate
- Clobetasol + Ofloxacin + Miconazole + Zinc Sulphate
- Clobetasol Propionate + Ofloxacin + Ornidazole + Terbinafine
- Clobetasole + Gentamicin + Miconazole + Zinc Sulphate
- Clomifene Citrate + Ubidecarenone + Zinc + Folic Acid + Methylcobalamin + Pyridoxine + Lycopene+ Selenium + Levocarnitine Tartrate + L-Arginine
- Clotrimazole + Beclomethasone + Lignocaine + Ofloxacin + Acetic Aicd + Sodium Methyl Paraben +Propyl Paraben
- Clotrimazole + Beclomethasone + Ofloxacin + Lignocaine
- Clotrimazole + Ofloxaxin + Lignocaine + Glycerine and Propylene Glycol
- Codeine + Chlorpheniramine + Alcohol Syrup
- Codeine + Levocetirizine + Menthol
- Combikit of 3 tablets of Serratiopeptidase (enteric coated 20000 units) + Diclofenac
- Potassium & 2 tablets of D
- Combikit of Azithromycin, Secnidazole and Fluconazole
- Combikit of Fluconazole Tablet, Azithromycin Tablet and Ornidazole Tablets
- Cyproheptadine + Thiamine
- Dextrometharphan + Chlopheniramine + Ammonium Chloride+ Menthol
- Dextrometharphan + Chlopheniramine + Ammonium + Sodium Citrate + Menthol
- Dextrometharphan + Phenylephrine + Guaiphenesin
- Dextromethophan + Chlopheniramine + Bromhexine
- Dextromethorphan + Ambroxol + Ammonium Chloride + Chlorpheniramine + Menthol
- Dextromethorphan + Ambroxol + Guaifenesin + Phenylephrine + Chlorpheniramine
- Dextromethorphan + Bromhexine + Guaiphenesin
- Dextromethorphan + Bromhexine + Guaiphenesin + Menthol
- Dextromethorphan + Cetirizine
- Dextromethorphan + Cetirizine + Guaifenesin + Ammonium Chloride
- Dextromethorphan + Chlorpheniramine + Chlorpheniramine Maleate
- Dextromethorphan + Chlorpheniramine + Guaiphenesin
- Dextromethorphan + Levocetirizine + Phenylephrine + Zinc
- Dextromethorphan + Paracetamol + Cetirizine + Phenylephrine
- Dextromethorphan + Phenylephrine + Ammonium Chloride + Menthol
- Dextromethorphan + Phenylephrine + Bromhexine + Guaifenesin + Chlorpheniramine
- Dextromethorphan + Phenylephrine + Cetirizine + Paracetamol + Caffeine
- Dextromethorphan + Phenylephrine + Cetirizine + Zinc + Menthol
- Dextromethorphan + Phenylephrine + Guaifenesin + Certirizine + Acetaminophen
- Dextromethorphan + Phenylephrine + Guaifenesin + Triprolidine
- Dextromethorphan + Phenylephrine + Tripolidine + Menthol
- Dextromethorphan + Phenylephrine + Zinc Gluconate + Menthol
- Dextromethorphan + Tripolidine + Phenylephirine
- Dextromethorphan + Triprolidine + Phenylephrine
- Dextromethorphen + Bromhexine + Chlorpheniramine Maleate + Guaiphenesin
- Dextromethorphen + Promethazine
- Diclofenac + paracetamol + chlorpheniramine maleate + mgnesium Trisillicate
- Diclofenac + Paracetamol + Chlorzoxazone + Famotidine
- Diclofenac + paracetamol + magnesium Trisilicate
- Diclofenac + Paracetamol injection
- Diclofenac + Tramadol + Chlorzoxazone
- Diclofenac + Tramadol + Paracetamol
- Diclofenac + Zinc Carnosine
- Dicyclomine + Paracetamol + Domperidone
- Diethyl Carbamazine + Chlorpheniramine + Guaifenesin
- Diethylcabamazine Citrate + Cetirizine + Guaiphenesin
- Diethylcarbamazine + Cetirizine + Ambroxol
- Diphenhydramine + Guaifenesin + Bromhexine + Ammonium Chloride + Menthol
- Diphenhydramine + Guaiphenesin + Ammonium Chloride + Bromhexine
- Diphenhydramine + Terpine + Ammonium Chloride + Sodium Chloride + Menthol
- Diphenoxylate + Atropine + Furazolidonee
- Disodium Hydrogen Citrate + Paracetamol
- Doxycycline + Serratiopeptidase
- Doxylamine + Pyridoxine + Mefenamic Acid + Paracetamol
- Dried Alumnium Hydroxie Gel + Prophantheline + Diazepam
- Drotaverine + Clidinium + Chlordiazepoxide
- Enrofloxacin + Bromhexin
- Ergotamine Tartrate + Belladona Dry Extarct+Caffeine + Paracetamol
- Ethylmorphine + Noscapine + Chlorpheniramine
- Famotidine + Oxytacaine + Magaldrate
- Flunarizine + Paracetamole + Domperidone
- Flupentixol + Escitalopram
- Furazolidone + Metronidazole + Loperamide
- Gabapentin + Mecobalamin + Pyridoxine + Thiamine
- Gentamicin Sulphate + Clotrimazole + Betamethasone + Lignocaine
- Gentamycin + Dexamethasone + Chloramphenicol + Tobramycin + Ofloxacin
- Glibenclamide + Metformin (SR)+ Pioglitazone
- Gliclazide 40mg + Metformin 400mg
- Gliclazide 80 mg + Metformin 325 mg
- Glimepiride + Pioglitazone + Metformin
- Glimepiride 1mg/2mg/3mg + Pioglitazone 15mg/15mg/15mg + Metformin 1000mg/1000mg/1000mg
- Glimepiride 1mg/2mg+ Pioglitazone 15mg/15mg + Metformin 850mg/850mg
- Glipizide 2.5mg + Metformin 400 mg
- Glucosamine + Methyl Sullfonyl Methane + Vitamin D3 + Manganese + Boron + Copper + Zinc
- Guaifenesin + Bromhexine + Chlorpheniramine + Paracetamol
- Guaifenesin + Bromhexine + Chlorpheniramine + Phenylephrine + Paracetamol + Serratiopeptidase(as enteric coated granules) 10000 SP Units
- Guaifenesin + Dextromethorphan
- Guaifenesin + Diphenhydramine + Bromhexine + Phenylephrine
- Heparin + Diclofenac
- Imipramine + Chlordiazepoxide + Trifluoperazine + Trihexyphenidyl
- Ketoconazole + Tea Tree oil + Allantion + Zinc Oxide + Aloe Vera + Jojoba oil +Lavander oil + Soa noodels
- Ketotifen + Cetirizine
- Ketotifen + Levocetrizine
- Ketotifen + Theophylline
- L-5-Methyltetrahydrofolate Calcium + Escitalopram
- L-Arginine + Sildenafil
- Levocetirizine + Ambroxol + Phenylephrine + Guaiphenesin
- Levocetirizine + Ambroxol + Phenylephrine + Paracetamol
- Levocetirizine + Dextromethorphan + Zinc
- Levocetirizine + Montelukast + Acebrophylline
- Levocetirizine + Paracetamol + Phenylephirine + Caffeine
- Levocetirizine + Phenylephrine + Ambroxol + Guaiphenesin + Paracetamol
- Levocetirizine + Ranitidine
- Levofloxacin + Bromhexine
- Levofloxacin + Ornidazole + Alpha Tocopherol Acetate
- Levothyroxine + Phyridoxine + Nicotinamide
- Lignocaine + Clotrimazole + Ofloxacin + Beclomethasone
- Lornoxicam + Paracetamol + serratiopeptidase
- Lornoxicam + paracetamol + Tramadol
- Lornoxicam + paracetamol + trypsin
- Magaldrate + Famotidine + Simethicone
- Magaldrate + Papain + Fungul Diastase + Simethicone
- Magaldrate + Ranitidine + Pancreatin + Domperidone
- Mebeverine & Inner HPMC capsule (Streptococcus Faecalis + Clostridium butyricum +
- Bacillusmesentricus + Lactic Acid Bacillus)
- Menthol + Anesthetic Ether
- Metformin (SR) 500mg + Pioglitazone 5mg
- Metformin (Sustainded Release) 500mg + Pioglitazone 15 mg + Glimepiride 3mg
- Metformin + Atorvastatin
- Metformin + Bromocriptine
- Metformin + Gliclazide + Chromium Polynicotinate
- Metformin + Gliclazide + Piogllitazone + Chromium Polynicotinate
- Metformin + Glimepiride + Methylcobalamin
- Metformin 1000/1000/500/500mg + Pioglitazone 7.5/7.5/7.5/7.5mg + Glimepiride 1/2/1/2mg
- Metformin 500mg/500mg+Gliclazide SR 30mg/60mg + Pioglitazone 7.5mg/7.5mg
- Metformin 850mg + Pioglitazone 7.5 mg + Glimepiride 1mg
- Metformin 850mg + Pioglitazone 7.5 mg + Glimepiride 2mg
- Metformin ER + Gliclazide MR + Voglibose
- Metronidazole + Norfloxacin
- Metronidazole + Tetracycline
- N-Acetyl Cysteine + Ambroxol + Phenylephrine + Levocertirizine
- Naphazoline + Carboxy Methyl Cellulose + Menthol + Camphor + Phenylephrine
- Naphazoline + Chlorpheniramine + Zinc Sulphate + Boric Acid + Sodium Chloride + Chlorobutol
- Naproxen + Paracetamol
- Neomycin + Doxycycline
- Nimesulide + Certirizine + Phenylephrine
- Nimesulide + Cetrizine + Caffeine
- Nimesulide + Diclofenac
- Nimesulide + Dicyclomine
- Nimesulide + Loratadine + Phenylephrine + Ambroxol
- Nimesulide + Paracetamol + Cetirizine + Phenylephrine
- Nimesulide + Paracetamol + Levocetirizine + Phenylephrine + Caffeine
- Nimesulide + paracetamol Injection
- Nimesulide + Paracetamol Suspension
- Nimesulide + Phenylephrine + Caffeine + Levocetirizine
- Nimesulide + Pitofenone + Fenpiverinium + Benzyl Alcohol
- Nimesulide + Serratiopeptidase
- Nimesulide + Tizanidine
- Nimorazole + Ofloxacin
- Norfloxacin+ Metronidazole + Zinc Acetate
- Oflaxacin + Ornidazole Suspension
- Ofloxacin + Clotrimazole + Betamethasone + Lignocaine
- Ofloxacin + Metronidazole + Zinc Acetate
- Ofloxacin + Nitazoxanide
- Ofloxacin + Ornidazole + Zinc Bisglycinate
- Olmesartan + Hydrochlorothiazide + Chlorthalidone
- Omepraozle + Paracetmaol+ Diclofenac
- Oxetacaine + Magaldrate + Famotidine
- Pantoprazole (as Enteric Coated Tablet) + Zinc Carnosine (as Film Coated Tablets
- Paracetamol + Ambroxol + Phenylephrine + Chlorpheniramine
- Paracetamol + Caffeine + Codeine
- Paracetamol + Caffine + Phenylephrine + Chlorpheniramine
- Paracetamol + Cetrizine + Caffeine
- Paracetamol + Chlorpheniramine + Ambroxol + Guaifenesin + Phenylephrine
- Paracetamol + Codeine + Chlorpheniramine
- Paracetamol + Dextromethorphan + Bromhexine + Phenylephrine + Diphenhydramine
- Paracetamol + Dextromethorphan + Chlorpheniramine
- Paracetamol + Diclofenac + famotidine
- Paracetamol + Disodium Hydrogen Citrate + Caffeine
- Paracetamol + DL Methionine
- Paracetamol + domperidone + Caffeine
- Paracetamol + Levocetirizine + Phenylephirine + Zink Gluconate
- Paracetamol + Levocetirizine + Pseudoephedrine
- Paracetamol + Loratadine + Dextromethophan + Pseudoepheridine + Caffeine
- Paracetamol + Loratadine + Phenylephrine + Dextromethorphan + Caffeine
- Paracetamol + mefenamic Acid + ranitidine + Dicyclomine
- Paracetamol + Pheniramine
- Paracetamol + Phenylephrine + Chlorpheneramine + Dextromethorphan + caffeine
- Paracetamol + Phenylephrine + Chlorpheniramine + Zinc Gluconate
- Paracetamol + Phenylephrine + Desloratadine + Zinc Gluconate + Ambroxol
- Paracetamol + Phenylephrine + Levocetirizine + Caffeine
- Paracetamol + Phenylephrine + Triprolidine
- Paracetamol + Phenylephrine + Triprolidine + Caffeine
- Paracetamol + Prochloperazine
- Paracetamol + Prochlorperazine Maleate
- Paracetamol + Promethazine
- Paracetamol + Propyphenazone + Caffeine
- Paracetamol + Pseudoephedrine + Cetrizine
- Paracetamol + Pseudoephedrine + Dextromethorphan + Cetirizine
- Paracetamol + Tapentadol
- Paracetamol+Phenylephrine+Levocetirizine+Sodium Citrate
- Paracetamol+Pseudoephedrine+Certirizine+Caffeine
- Permethrin + Cetrimide + Menthol
- phenylbutazone + sodium salicylate
- Phenylephrine + Chlorpheniramine + Paracetamol + Bromhexine + Caffeine
- Pholcodine + Phenylephrine + Promethazine
- Pioglitazone 15mg + Metformin 850 mg
- Pioglitazone 30 mg + Metformin 500 mg
- Pioglitazone 7.5/7.5mg + Metformin 500/1000mg
- Pseudoephedrine + Bromhexine
- Pseudoephedrine + Cetirizine
- Pseudoephedrine + Dextromethorphan + Cetirizine
- Rabeprazole + Diclofenac + Paracetamol
- Rabeprazole + Zinc + Domperidone
- Rabeprazole + Zinc Carnosine
- Ranitidine + Domperidone + Simethicone
- Ranitidine + Magaldrate
- Ranitidine + Magaldrate + Simethicone
- Roxithromycin + Serratiopeptidase
- Salbutamol + Aminophylline + Guaiphensin
- Salbutamol + Bromhexine + Guaiphenesin + Menthol
- Salbutamol + Certirizine + Ambroxol
- Salbutamol + Choline Theophylinate + Ambroxol
- Salbutamol + Choline Theophylinate + Carbocisteine
- Salbutamol + Theophylline + Bromhexine
- Salbutamol+Hydroxyethyltheophylline (Etofylline) + Bromhexine
- Sildenafil + Estradiol Valerate
- Tamsulosin + Diclofenac
- Telmisartan + Metformin
- Terbutaline + Ambroxol + Guaiphenesin + Zinc + Menthol
- Terbutaline + Bromhexine + Etofylline
- Terbutaline + Bromhexine + Guaiphenesin + Dextromethorphan
- Terbutaline + Etofylline + Ambroxol
- Terbutaline + N-Acetyl L-Cysteine + Guaifenesin
- Terpinhydrate + Dextromethorphan + Menthol
- Thyroid + Thiamine + Riboflavin + Phyridoxine + Calcium Pantothenate + Tocopheryl Acetate +Nicotinamide
- Thyroxine + Pyridoxine + Folic Acid
- Tranexamic Acid + Proanthocyanidin
- Ursodeoxycholic Acid + Silymarin
- Voglibose + Pioglitazone + Metformin
- Voglibose+ Metformin + Chromium Picolinate
- Zinc Carnosine + Magnesium Hydroxide + Dried Aluminium Hydroxide + Simethicone
- Zinc Carnosine + Oxetacaine
- Zinc Carnosine + Sucralfate
Reference : National Health Portal