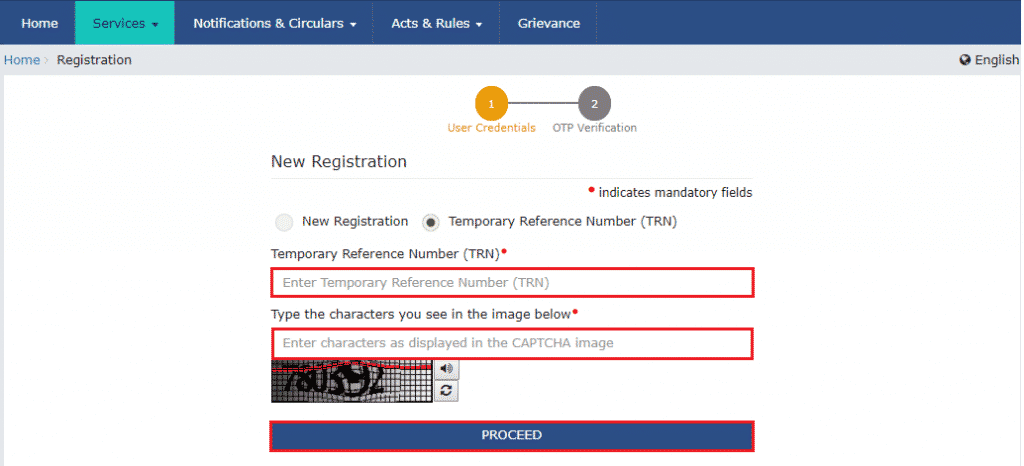
Latest Notification on DPCO Price List
Latest Notification on DPCO Price List
ORDER
S.O. 1462(E) In exercise of the powers, conferred by paragraph 4, 6, 10, 11, 14, 16, 17 and 18 of the Drugs (Prices Control) Order, 2013, read with S.O. No. 1394(E) dated the 30th May 2013 issued by the Government of India in the Ministry of Chemicals and Fertilizers, and in supersession of the Order(s) of the Government of India in the Ministry of Chemicals and Fertilizers (National Pharmaceutical Pricing Authority) S.O. Number and date specified in column no. 6(a) & 6(b) mentioned in the table below, the National Pharmaceutical Pricing Authority, hereby fixes the prices as specified in column (5) of the table hereinbelow as ceiling prices exclusive of goods and services tax applicable, if any in respect of the Scheduled formulations specified in the corresponding entry in column (2) of the said Table with the dosage form & strength and unit specified respectively in the corresponding entries in columns (3) and (4) thereof:
Table
Price Revision as per Annual Wholesale Price Index (WPI) @ 3.43812% increase
| SlNo | Medicines | Dosage form andStrength | Unit | Ceilingprice (wef 01.4.2018
with WPI @ 3.43812%) |
Existing S.O. No. &Date | |
| (1) | (2) | (3) | (4) | (5) | 6(a) | 6(b) |
| 1 | Acetyl Salicylic Acid | Tablet 300mg | 1 Tablet | 0.21243 | 2060(E) | 30.06.2017 |
| 2 | Calcium carbonate | Tablet 250 mg | 1 Tablet | 1.83 | 2060(E) | 30.06.2017 |
| 3 | Calcium carbonate | Tablet 500 mg | 1 Tablet | 1.82 | 2060(E) | 30.06.2017 |
| 4 | Condoms | 1 Condom | 8.56 | 2061(E) | 30.06.2017 | |
| 5 | Dapsone | Tablet 50 mg | 1 Tablet | 0.22150 | 2061(E) | 30.06.2017 |
| 6 | Daunorubicin | Injection 20 mg | Each Pack | 400.74 | 2061(E) | 30.06.2017 |
| 7 | Ethyl Alcohol 70%Solution | 1 ML | 0.54 | 2060(E) | 30.06.2017 | |
| 8 | Etoposide | Capsules 100 mg | 1 Capsule | 56.83 | 2060(E) | 30.06.2017 |
| 9 | Framycetin Sulphate | Cream 0.50% | 1 GM | 0.82 | 2060(E) | 30.06.2017 |
| 10 | Gentian Violet Paint | 1% | 1 ML | 0.06133 | 2060(E) | 30.06.2017 |
| 11 | Isoniazid | Syrup 100 mg/5ml | 1 ML | 0.24260 | 2061(E) | 30.06.2017 |
| 12 | Medroxy Progesterone Acetate | Tablet 5mg | 1 Tablet | 2.8 | 2060(E) | 30.06.2017 |
| 13 | Nitrous Oxide Inhalation | Cubic Meter | 224.05 | 2060(E) | 30.06.2017 | |
| 14 | Oral Poliomyelitisvaccine (LA) | Solution | 1 ML | 113.19 | 2061(E) | 30.06.2017 |
| 15 | Oxygen Inhalation | Cubic Meter | 17.19 | 2060(E) | 30.06.2017 | |
| 16 | Praziquantel | Tablet 600 mg | 1 Tablet | 27.06 | 2061(E) | 30.06.2017 |
| 17 | Rifampicin | Tablet 450mg | 1 Tablet | 4.16 | 2060(E) | 30.06.2017 |
| 18 | Sodium Nitrite | Injection 30mg/ml | 1 ML | 20.25 | 2060(E) | 30.06.2017 |
Notes:-
- The ceiling prices are applicable with effect from 4.2018 (ceiling prices are inclusive of Wholesale Price Index (WPI) @3.43812% for the year 2017 over 2016).
- All manufacturers of scheduled formulations, selling the branded or generic or both the versions of scheduled formulations at a price higher than the ceiling price (plus goods and services taxes as applicable) so fixed and notified by the Government, shall revise the prices of all such formulations downward not exceeding the ceiling price specified in column (5) in the above table plus goods and services taxes as applicable, if
- All the existing manufacturers of above mentioned scheduled formulations having MRP lower than the ceiling price specified in column (5) in the above table (plus goods and services taxes as applicable, if any), may revise the existing R.P. of their formulations, on the basis of WPI @ 3.43812% for year 2017 in accordance with paragraph 16(2) of DPCO, 2013, read with para 13(2) of DPCO, 2013.
- The manufacturers may add goods and services taxes only if they have paid actually or if it is payable to the Government on the ceiling price mentioned in column (5) of the above said
- The ceiling price for a pack of the scheduled formulation shall be arrived at by the concerned
manufacturer in accordance with the ceiling price specified in column (5) of the above table as per provisions contained in paragraph 11 of the Drugs (Prices Control) Order, 2013. The manufacturer shall issue a price list in Form–V from date of Notification as per paragraph 24 of the DPCO, 2013 to NPPA through IPDMS and submit a copy to State Drug Controller and dealers.
- As per para 24(4) of DPCO 2013, every retailer and dealer shall display price list and the supplementary price list, if any, as furnished by the manufacturer, on a conspicuous part of the premises where he carries on business in a manner so as to be easily accessible to any person wishing to consult the
- Where an existing manufacturer of scheduled formulation with dosage or strength or both as specified in the above table launches a new drug as per paragraph 2 (u) of the DPCO, 2013 such existing manufacturer shall apply for prior price approval of such new drug to the NPPA in Form I as specified under Schedule-II of the DPCO,
- The manufacturers of above said scheduled formulations shall furnish quarterly return to the NPPA, in respect of production / import and sale of scheduled formulations in Form-III of Schedule-II of the DPCO, 2013 through Any manufacturer intending to discontinue production of above said scheduled formulation shall furnish information to the NPPA, in respect of discontinuation of production and/or import of scheduled formulation in Form-IV of Schedule-II of the DPCO, 2013 at least six months prior to the intended date of discontinuation.
- The manufacturers not complying with the ceiling price and notes specified hereinabove shall be liable to deposit the overcharged amount along with interest thereon under the provisions of the Drugs (Prices Control) Order, 2013 read with Essential Commodities Act,
- Consequent to the issue of ceiling prices of such formulations as specified in column (2) of the above table in this notification, the price order(s) fixing ceiling or retail price, if any, issued prior to the above said date of notification, stand(s) superseded.
2nd April 2018
ORDER
S.O. 1463(E) In exercise of powers, conferred by subparagraph (3) and (4) of paragraph 11 and paragraph 14 of the Drugs (Prices Control) Order, 2013, read with S.O. 1394(E) dated the 30th May 2013, S.O. 1192(E) dated 22nd March 2016 issued by the Government of India in the Ministry of Chemicals and Fertilizers and in supersession of the order of the Government of India in the Ministry of Chemicals and Fertilizers (National Pharmaceutical Pricing Authority) No. S.O 1993(E) dated 3rd June 2016 read with Corrigendum order S.O 2062 dated 9th June 2016, S.O 2210(E) dated 24th June 2016, S.O 2578(E) dated 1st August 2016, S.O. 3161(E) dated 6th October 2016, S.O. 1051(E) dated 1st April 2017 and
S.O. 2062(E) dated 30th June 2017 in so far as they relate to formulation packs of Non-Glass with special features (mentioned as Non-PVC in S.O. 1993(E) dated 3rd June 2016) mentioned in the Table A hereinbelow, manufactured by the manufacturers specified in Table B for specified products and pack-sizes, except in respect of things done or omitted to be done before such supersession, the National Pharmaceutical Pricing Authority, hereby revises the price based on Wholesale price index(WPI) of 2017 as specified in column (5) of the Table A herein below as separate ceiling price exclusive of goods and services tax applicable, if any in respect of the scheduled formulations specified in the corresponding entry in column (2) of the said Table with the dosage form and strength and unit/packaging specified respectively in the corresponding entries in columns (3) and (4) thereof:
Table A: Price Revision as per Annual Wholesale Price Index (WPI) @ 3.43812% increase.
| Sl No | Medicines | Dosage form andStrength | Unit/packaging | Ceiling price(wef 01.4.2017
with WPI @ 3.43812%) |
| (1) | (2) | (3) | (4) | (5) |
| 1 | Glucose | Injection 5% | 1000ml Non-Glass withspecial features | 71.70 |
| 2 | Glucose | Injection 5% | 500ml Non-Glass withspecial features | 62.03 |
| 3 | Glucose (A) +Sodium Chloride (B) | Injection 5% (A) +0.9% (B) | 1000ml Non-Glass withspecial features | 75.35 |
| 4 | Glucose (A) +Sodium Chloride (B) | Injection 5% (A) +0.9% (B) | 500ml Non-Glass with special features | 64.47 |
| 5 | Sodium Chloride | Injection 0.9% | 100ml Non-Glass withspecial features | 31.71 |
| 6 | Sodium Chloride | Injection 0.9% | 250ml Non-Glass withspecial features | 46.82 |
| 7 | Sodium Chloride | Injection 0.9% | 500ml Non-Glass withspecial features | 66.30 |
| 8 | Glucose (A) +Sodium Chloride (B) | Injection 5% (A) +0.9% (B) | 1000ml Non-Glass with special features | 74.26 |
TABLE ‘B’
| Sl. No. | Name of Manufacturer | Product /Brand Name |
| (1) | (2) | (3) |
| 1 | M/s B.Braun Medical (I) Pvt Ltd. | Ecoflac Plus bottle with Euro head |
| 2 | M/s Amanta Healthcare Ltd. | Steriport bottle |
| 3 | M/s Aculife Healthcare Pvt Ltd. | Aculife bottle with Euro head |
| 4 | M/s Albert David Limited | Albert David bottle with Euro head |
| 5 | M/s Denis Chem Limited | Aquapulse with Euro head |
| 6 | M/s Claris Life Sciences Limited | Claris bottle with Euro head |
| 7 | M/s Fresenius Kabi India Pvt Limited | Freeflex bags |
| 8 | M/s Otsuka Pharmaceutical India Private Ltd. (previously known as Claris Otsuka Private Limited) | Unibag |
| 9 | M/s Aishwarya Lifesciences | Lifusion Euro head bottle |
| 10 | M/s Baxter (India) Pvt. Ltd. | Viaflex bags |
| 11 | M/s Otsuka Pharmaceutical India Private Ltd.(previously known as Claris Otsuka Private Limited) | Euro head bottle |
| 12 | M/s Fresenius Kabi India Pvt Limited | Euro head bottle |
- The ceiling prices are applicable with effect from 4.2018 (ceiling prices are inclusive of Wholesale Price Index (WPI) @3.43812% for the year 2017 over 2016).
- The manufacturers of scheduled formulations, selling abovesaid products/brand name of
scheduled formulations at price higher than the ceiling price (plus goods and services tax as applicable) so fixed and notified by the Government, shall revise the prices of all such formulations downward not exceeding the ceiling price specified in column (5) in the above table plus goods and services tax as applicable, if any.
- The manufacturers of above mentioned scheduled formulations having MRP lower than the ceiling price specified in column (5) in the above table (plus goods and services tax as applicable, if any), may revise the existing R.P. of their formulations , on the basis of WPI @ 3.43812% for year 2017 in accordance with paragraph 16(2) of DPCO, 2013, read with para 13(2) of DPCO, 2013.
- The manufacturers may add goods and services tax only if they have paid actually or if it is payable to the Government on the ceiling price mentioned in column (5) of the above said
- Any other manufacturer claiming separate ceiling price for Non-Glass with special feature shall apply to NPPA for separate ceiling price approval with details and demonstrate, that such pack has all of the features as (i) self collapsibility and self- sealability (ii) not having air-vent; and (iii) there is no chance of contamination during manufacture/ infusion/ admixing levels along with documentation and
- For other special features claimed or any other pack size manufactured, the manufacturer shall approach the NPPA for specific price approval for its formulation
- The ceiling price for a pack of the scheduled formulation shall be arrived at by the
concerned manufacturer in accordance with the ceiling price specified in column (5) of the above table as per provisions contained in paragraph 11 of the Drugs (Prices Control) Order, 2013. The manufacturer shall issue a price list in Form–V from date of Notification as per paragraph 24 of the DPCO, 2013 to NPPA through IPDMS and submit a copy to State Drug Controller and dealers.
- As per para 24(4) of DPCO 2013, every retailer and dealer shall display price list and the supplementary price list, if any, as furnished by the manufacturer, on a conspicuous part of the premises where he carries on business in a manner so as to be easily accessible to any person wishing to consult the
- Where an existing manufacturer of scheduled formulation with dosage or strength or both as specified in the above table launches a new drug as per paragraph 2 (u) of the DPCO, 2013 such existing manufacturer shall apply for prior price approval of such new drug to the NPPA in Form I as specified under Schedule-II of the DPCO,
- The manufacturers of above said scheduled formulations shall furnish quarterly return to the NPPA, in respect of production/import and sale of scheduled formulations in Form -III of Schedule-II of the DPCO, 2013 through Any manufacturer intending to discontinue production of above said scheduled formulation shall furnish information to the NPPA, in respect of discontinuation of production and/or import of scheduled formulation in Form-IV of Schedule-II of the DPCO, 2013 at least six months prior to the intended date of discontinuation.
- The manufacturers not complying with the ceiling price and notes specified hereinabove shall be liable to deposit the overcharged amount along with interest thereon under the provisions of the Drugs (Prices Control) Order, 2013 read with Essential Commodities Act,
- Consequent to the issue of ceiling prices of such formulations as specified in column (2) of the above table in this notification, the price order(s) fixing ceiling or retail price, if any, issued prior to the above said date of notification, stand(s)
2nd April 2018
ORDER
SO 1464(E) In partial modification of the notification issued by National Pharmaceutical Pricing Authority, Department of Pharmaceuticals, Ministry of Chemical and Fertilizers vide S.O. 639(E) dated 12.02.2018, regarding the fixation of ceiling price of the coronary stents, after considering the Wholesale Price Index (WPI) @ 3.43812 percent for the year 2017 over 2016, It has been decided to revise the ceiling prices exclusive of goods and services tax applicable with effect from 01.04.2018 as mentioned in the table in para 13 of the original notification as follows:
TABLE
| Sl No | Coronary Stents( Sl 31 in Schedule I of DPCO 2013) | Unit(in Number) | Ceiling Price(in Rs.) |
| (1) | (2) | (3) | (4) |
| 1 | Bare Metal Stents | 1 | 7,923 |
| 2 | Drug Eluting Stents (DES) including metallic DESand Bioresorbable Vascular Scaffold (BVS)/ Biodegradable Stents | 1 | 28,849 |
All the terms and conditions/notes as mentioned in the original order dated 12.02.2018 thereto shall remain same.2nd April 2018
ORDER
S.O. 1461(E) In exercise of the powers, conferred by paragraph 4, 6, 10, 11, 14, 16, 17 and 18 of the Drugs (Prices Control) Order, 2013, read with S.O. No. 1394(E) dated the 30th May 2013 issued by the Government of India in the Ministry of Chemicals and Fertilizers, and in supersession of the Order(s) of the Government of India in the Ministry of Chemicals and Fertilizers (National Pharmaceutical Pricing Authority) S.O. Number and date specified in column no. 6(a) & 6(b) mentioned in the table below, the National Pharmaceutical Pricing Authority, hereby fixes the prices as specified in column (5) of the table herein below as ceiling prices exclusive of goods and services tax applicable, if any in respect of the Scheduled formulations specified in the corresponding entry in column (2) of the said Table with the dosage form & strength and unit specified respectively in the corresponding entries in columns (3) and (4) thereof:
Table: Price Revision as per Annual Wholesale Price Index (WPI) @ 3.43812% increase.
| SlNo | Medicines | Dosage form andStrength | Unit | Ceilingprice (wef 1.4.2018
with WPI @ 3.43812%) |
Existing S.O. No. &Date | |
| (1) | (2) | (3) | (4) | (5) | 6(a) | 6(b) |
| 1 | 5-aminosalicylic Acid | Suppository 500mg Retention Enema | 1 Suppository | 15.87 | 2058(E) | 30.06.2017 |
| 2 | 5-aminosalicylic Acid | Tablet 400 mg | 1 Tablet | 7.01 | 2058(E) | 30.06.2017 |
| 3 | 5-Fluorouracil | Injection 250mg/5 ml | 1 ml | 2.20 | 2059(E) | 30.06.2017 |
| 4 | 6-Mercaptopurine | Tablet 50mg | 1 Tablet | 6.06 | 2398(E) | 28.07.2017 |
| 5 | Abacavir | Tablet 300 mg | 1 Tablet | 44.26 | 2058(E) | 30.06.2017 |
| 6 | Abacavir (A) + Lamivudine (B) | Tablet 60 mg(A)+ 30 mg(B) | 1 Tablet | 18.85 | 2400(E) | 28.07.2017 |
| 7 | Abacavir (A) + Lamivudine (B) | Tablet 600mg(A)+ 300
mg(B) |
1 Tablet | 85.53 | 2400(E) | 28.07.2017 |
| 8 | Acetazolamide | Capsule 250 mg | 1 Capsule | 4.25 | 2059(E) | 30.06.2017 |
| 9 | Acetazolamide | Tablet 250 mg | 1 Tablet | 3.43 | 2059(E) | 30.06.2017 |
| 10 | Acetylsalicylic acid | Effervescent/ Dispersible/ Enteric coatedTablet 100 mg | 1 Tablet | 0.16809 | 3722(E) | 23.11.2017 |
| 11 | Acetylsalicylic acid | Effervescent/ Dispersible/ Enteric coatedTablet 150 mg | 1 Tablet | 1.13 | 2058(E) | 30.06.2017 |
| 12 | Acetylsalicylic acid | Effervescent/ Dispersible/ Enteric coatedTablet 75 mg | 1 Tablet | 1.15 | 2058(E) | 30.06.2017 |
| 13 | Acetylsalicylic acid | Tablet 100 mg | 1 Tablet | 0.16730 | 2058(E) | 30.06.2017 |
| 14 | Acetylsalicylic acid | Tablet 150 mg | 1 Tablet | 0.38 | 2058(E) | 30.06.2017 |
| 15 | Acetylsalicylic acid | Tablet 325mg | 1 Tablet | 0.52 | 3723(E) | 23.11.2017 |
| 16 | Acetylsalicylic acid | Tablet 350mg | 1 Tablet | 0.29 | 3091(E) | 20.09.2017 |
| 17 | Acetylsalicylic acid | Tablet 75 mg | 1 Tablet | 0.29 | 2058(E) | 30.06.2017 |
| 18 | Actinomycin D | Powder forInjection 0.5mg | Each Pack | 297.65 | 3089(E) | 20.09.2017 |
| 19 | Acyclovir | Ointment 3% | 1 gm | 9.77 | 2058(E) | 30.06.2017 |
| 20 | Acyclovir | Oral Liquid 400 mg/5ml | 1 ml | 1.19 | 2058(E) | 30.06.2017 |
| 21 | Acyclovir | Tablet 200 mg | 1 Tablet | 6.36 | 2058(E) | 30.06.2017 |
| 22 | Acyclovir | Tablet 400 mg | 1 Tablet | 11.55 | 2058(E) | 30.06.2017 |
| 23 | Acyclovir | Powder forInjection 250 mg | Each Pack | 333.49 | 2058(E) | 30.06.2017 |
| 24 | Acyclovir | Powder forInjection 500 mg | Each Pack | 430.74 | 2058(E) | 30.06.2017 |
| 25 | Adenosine | Injection 3mg/ml | 1 ml | 92.41 | 650(E) | 13.02.2018 |
| 26 | Adrenaline | Injection 1 mg/ml | 1 ml | 14.69 | 2058(E) | 30.06.2017 |
| 27 | Albendazole | Oral Liquid 200mg/5ml | 1 ml | 1.50 | 2058(E) | 30.06.2017 |
| 28 | Albendazole | Tablet 400 mg | 1 Tablet | 7.23 | 2058(E) | 30.06.2017 |
| 29 | Allopurinol | Tablet 100 mg | 1 Tablet | 1.78 | 2058(E) | 30.06.2017 |
| 30 | Allopurinol | Tablet 300 mg | 1 Tablet | 7.02 | 2058(E) | 30.06.2017 |
| 31 | Alprostadil | Injection 0.5 mg/ml | 1 ml | 5,315.42 | 2058(E) | 30.06.2017 |
| 32 | Alteplase | Powder forInjection 20 mg | Each Pack | 17,827.56 | 3724(E) | 23.11.2017 |
| 33 | Alteplase | Powder for Injection 50 mg | Each Pack | 37,222.21 | 3724(E) | 23.11.2017 |
| 34 | Amiodarone | Injection 50mg/ml | 1 ml | 18.40 | 2058(E) | 30.06.2017 |
| 35 | Amiodarone | Tablet 100 mg | 1 Tablet | 5.36 | 2058(E) | 30.06.2017 |
| 36 | Amiodarone | Tablet 200 mg | 1 Tablet | 10.63 | 2058(E) | 30.06.2017 |
| 37 | Amitriptyline | Tablet 10 mg | 1 Tablet | 2.15 | 2058(E) | 30.06.2017 |
| 38 | Amitriptyline | Tablet 25 mg | 1 Tablet | 2.12 | 2058(E) | 30.06.2017 |
| 39 | Amitriptyline | Tablet 50 mg | 1 Tablet | 5.45 | 2058(E) | 30.06.2017 |
| 40 | Amitriptyline | Tablet 75 mg | 1 Tablet | 5.27 | 2058(E) | 30.06.2017 |
| 41 | Amlodipine | Tablet 10 mg | 1 Tablet | 4.76 | 2058(E) | 30.06.2017 |
| 42 | Amlodipine | Tablet 2.5 mg | 1 Tablet | 1.55 | 2058(E) | 30.06.2017 |
| 43 | Amlodipine | Tablet 5mg | 1 Tablet | 2.45 | 2401(E) | 28.07.2017 |
| 44 | Amoxicillin | Capsule 250 mg | 1 Capsule | 2.07 | 2058(E) | 30.06.2017 |
| 45 | Amoxicillin | Oral Liquid 250 | 1 ml | 1.27 | 2058(E) | 30.06.2017 |
| mg/5ml | ||||||
| 46 | Amoxicillin | Capsule 500mg | 1 Capsule | 6.06 | 2401(E) | 28.07.2017 |
| 47 | Amoxicillin (A) + Clavulanic acid (B) | Dry Syrup 125mg(A) + 31.25
(B)/5 ml |
1 ml | 2.03 | 2058(E) | 30.06.2017 |
| 48 | Amoxicillin (A) + Clavulanicacid (B) | Oral Liquid 200mg(A) + 28.5
mg(B)/5ml |
1 ml | 1.70 | 2058(E) | 30.06.2017 |
| 49 | Amoxicillin (A) + Clavulanic acid (B) | Powder for Injection 500mg(A) + 100
mg(B) |
Each Pack | 84.50 | 2058(E) | 30.06.2017 |
| 50 | Amoxicillin (A) +Clavulanic acid (B) | Tablet 500mg(A)+125mg(B) | 1 Tablet | 16.86 | 2401(E) | 28.07.2017 |
| 51 | Amoxicillin (A) +Clavulanic acid (B) | Powder for Injection 1g (A)+200mg(B) | Each Pack | 117.87 | 2401(E) | 28.07.2017 |
| 52 | Amphotericin B - Lipid/Liposomal | Powder forInjection 50 mg | Each Pack | 3,519.53 | 269(E) | 16.01.2018 |
| 53 | Amphotericin B –Conventional | Powder forInjection 50 mg | Each Pack | 290.71 | 2059(E) | 30.06.2017 |
| 54 | Ampicillin | Powder forInjection 1 gm | Each Pack | 20.04 | 2058(E) | 30.06.2017 |
| 55 | Ampicillin | Powder for Injection 500 mg | Each Pack | 12.34 | 2058(E) | 30.06.2017 |
| 56 | Anti-D immunoglobulin | Injection 300mcg | Each Pack | 2,003.41 | 3723(E) | 23.11.2017 |
| 57 | Anti-D Immunoglobulin | Injection 150mcg | Each Vial | 1,561.59 | 3913(E) | 18.12.2017 |
| 58 | Anti-rabies immunoglobulin | Injection 150 IU/ml | 1 ml | 2,603.95 | 2059(E) | 30.06.2017 |
| 59 | Anti-rabies immunoglobulin | Injection 300IU/ml | 1 ml | 91.80 | 2059(E) | 30.06.2017 |
| 60 | Anti-tetanus immunoglobulin | Each Pack | 773.78 | 3090(E) | 20.09.2017 | |
| 61 | Arsenic Trioxide | Injection 1mg/ml | 1 ml | 50.97 | 2397(E) | 28.07.2017 |
| 62 | Artemether (A) + Lumefantrine (B) | Oral Liquid 80mg(A) +480
mg(B) /5ml |
1 ml | 3.79 | 2058(E) | 30.06.2017 |
| 63 | Artemether (A) +Lumefantrine (B) | Tablet 20 mg(A)+ 120 mg(B) | 1 Tablet | 11.75 | 2058(E) | 30.06.2017 |
| 64 | Artemether (A) +Lumefantrine (B) | Tablet 40 mg(A)+ 240 mg(B) | 1 Tablet | 15.14 | 2058(E) | 30.06.2017 |
| 65 | Artemether (A) +Lumefantrine (B) | Tablet 80 mg(A)+ 480 mg(B) | 1 Tablet | 21.74 | 2058(E) | 30.06.2017 |
| 66 | Artesunate | Powder forInjection 120 mg | Each Pack | 390.57 | 2059(E) | 30.06.2017 |
| 67 | Artesunate | Powder forInjection 60 mg | Each Pack | 205.15 | 2059(E) | 30.06.2017 |
| 68 | Artesunate (A) +Sulphadoxine - Pyrimethamine (B) | Tablet 100 mg(A)+ 1 Tablet (750 mg+ 37.5 mg) (B) | Combi pack | 27.69 | 3091(E) | 20.09.2017 |
| 69 | Artesunate (A) + Sulphadoxine -Pyrimethamine (B) | Tablet 150 mg(A)+ 2 Tablet (500 mg+ 25 mg) (B) | Combi pack | 37.59 | 3091(E) | 20.09.2017 |
| 70 | Artesunate (A) + Sulphadoxine - Pyrimethamine (B) | Tablet 200 mg(A)+ 2 Tablet (750 mg+ 37.5 mg) (B) | Combi pack | 34.27 | 3091(E) | 20.09.2017 |
| 71 | Artesunate (A) +Sulphadoxine - Pyrimethamine (B) | Tablet 25 mg(A)+ 1 Tablet (250 mg+ 12.5 mg) (B) | Combi pack | 18.45 | 3091(E) | 20.09.2017 |
| 72 | Artesunate (A) +Sulphadoxine - Pyrimethamine (B) | 1 Tablet 50mg(A) + 1 Tablet
(500 mg+ 25 mg) (B) |
Combi Pack | 19.92 | 2058(E) | 30.06.2017 |
| 73 | Ascorbic Acid (Vitamin C) | Tablet 100mg | 1 Tablet | 0.18208 | 3090(E) | 20.09.2017 |
| 74 | Ascorbic Acid (Vitamin C) | Tablet 500 mg | 1 Tablet | 0.85 | 2058(E) | 30.06.2017 |
| 75 | Atazanavir (A) + Ritonavir (B) | Tablet 300 mg(A)+ 100 mg(B) | 1 Tablet | 92.66 | 2400(E) | 28.07.2017 |
| 76 | Atenolol | Tablet 100 mg | 1 Tablet | 3.27 | 2058(E) | 30.06.2017 |
| 77 | Atenolol | Tablet 50 mg | 1 Tablet | 1.68 | 2058(E) | 30.06.2017 |
| 78 | Atorvastatin | Tablet 10 mg | 1 Tablet | 5.15 | 2058(E) | 30.06.2017 |
| 79 | Atorvastatin | Tablet 20 mg | 1 Tablet | 12.47 | 2058(E) | 30.06.2017 |
| 80 | Atorvastatin | Tablet 40 mg | 1 Tablet | 18.11 | 2058(E) | 30.06.2017 |
| 81 | Atracurium | Injection 10 mg/ml | Each Pack | 49.20 | 2058(E) | 30.06.2017 |
| 82 | Atropine | Ointment 1% | 1 gm | 3.55 | 2400(E) | 28.07.2017 |
| 83 | Atropine | Injection 0.6mg/ml | 1 ml | 3.93 | 2400(E) | 28.07.2017 |
| 84 | Atropine | Drops 1% | 1 ml | 3.22 | 2400(E) | 28.07.2017 |
| 85 | Azathioprine | Tablet 50 mg | 1 Tablet | 9.52 | 2059(E) | 30.06.2017 |
| 86 | Azithromycin | Capsule 250 mg | 1 Capsule | 9.86 | 2058(E) | 30.06.2017 |
| 87 | Azithromycin | Capsule 500 mg | 1 Capsule | 15.56 | 2058(E) | 30.06.2017 |
| 88 | Azithromycin | Oral Liquid 200 mg/5ml | 1 ml | 2.89 | 2058(E) | 30.06.2017 |
| 89 | Azithromycin | Tablet 250mg | 1 Tablet | 9.91 | 2401(E) | 28.07.2017 |
| 90 | Azithromycin | Tablet 500mg | 1 Tablet | 19.99 | 2401(E) | 28.07.2017 |
| 91 | Azithromycin | Powder for Injection 500mg | Each Pack | 180.45 | 2401(E) | 28.07.2017 |
| 92 | Baclofen | Tablet 10 mg | 1 Tablet | 10.09 | 2059(E) | 30.06.2017 |
| 93 | Baclofen | Tablet 20 mg | 1 Tablet | 13.89 | 2059(E) | 30.06.2017 |
| 94 | Baclofen | Tablet 5 mg | 1 Tablet | 5.20 | 2059(E) | 30.06.2017 |
| 95 | BCG vaccine | Each Dose | 4.47 | 3723(E) | 23.11.2017 | |
| 96 | Benzathine benzylpenicillin | Powder for Injection 12 lacunits | Each Pack | 11.40 | 2058(E) | 30.06.2017 |
| 97 | Benzathine benzylpenicillin | Powder forInjection 6 lac units | Each Pack | 7.55 | 2058(E) | 30.06.2017 |
| 98 | Benzoyl Peroxide | Cream 2.5% | 1 gm | 2.20 | 2058(E) | 30.06.2017 |
| 99 | Benzoyl Peroxide | Gel 2.5% | 1 gm | 3.52 | 2058(E) | 30.06.2017 |
| 100 | Benzyl penicillin | Powder for Injection 10 Lac Units | Each Pack | 4.88 | 2601(E) | 11.08.2017 |
| 101 | Betamethasone | Cream 0.05% | 1 gm | 0.55 | 2058(E) | 30.06.2017 |
| 102 | Betamethasone | Cream 0.1% | 1 GM | 0.79 | 2397(E) | 28.07.2017 |
| 103 | Betamethasone | Gel 0.05% | 1 gm | 0.50 | 2058(E) | 30.06.2017 |
| 104 | Betamethasone | Lotion 0.1% | 1 ml | 0.77 | 2397(E) | 28.07.2017 |
| 105 | Betamethasone | Lotion 0.5% | 1 ml | 0.50 | 2397(E) | 28.07.2017 |
| 106 | Betamethasone | Injection 4mg/ml | 1 ml | 3.94 | 2058(E) | 30.06.2017 |
| 107 | Bicalutamide | Tablet 50 mg | 1 Tablet | 64.39 | 2058(E) | 30.06.2017 |
| 108 | Bisacodyl | Suppository 5 mg | 1 Suppository | 7.91 | 2058(E) | 30.06.2017 |
| 109 | Bisacodyl | Tablet 5 mg | 1 Tablet | 0.94 | 2058(E) | 30.06.2017 |
| 110 | Bleomycin | Powder for Injection 15Units | Each Pack | 582.63 | 2059(E) | 30.06.2017 |
| 111 | Bortezomib | Powder forInjection 2 mg | Each Pack | 11,543.78 | 2058(E) | 30.06.2017 |
| 112 | Budesonide | Inhalation(MDI/DPI) 100
mcg/dose |
1 Dose | 1.23 | 2058(E) | 30.06.2017 |
| 113 | Budesonide | Inhalation (MDI/DPI) 200mcg/dose | 1 Dose | 1.52 | 2058(E) | 30.06.2017 |
| 114 | Budesonide | Nasal Spray 100 mcg/dose | 1 Dose | 0.85 | 2058(E) | 30.06.2017 |
| 115 | Budesonide | Nasal Spray 50 mcg/dose | 1 Dose | 0.95 | 2058(E) | 30.06.2017 |
| 116 | Budesonide | Respiratory Solution for use in Nebulizer 0.5mg/ml | 1 ml | 10.05 | 2058(E) | 30.06.2017 |
| 117 | Budesonide | Respiratory Solution for usein Nebulizer 1 mg/ml | 1 ml | 12.12 | 2058(E) | 30.06.2017 |
| 118 | Budesonide (A)+ Formoterol (B) | Inhalation (MDI/DPI) 100mcg (A) + 6 mcg
(B) |
1 Dose | 1.76 | 2058(E) | 30.06.2017 |
| 119 | Budesonide (A)+ Formoterol(B) | Inhalation(MDI/DPI) 200
mcg (A) + 6 mcg (B) |
1 Dose | 2.21 | 2058(E) | 30.06.2017 |
| 120 | Budesonide (A)+ Formoterol (B) | Inhalation (MDI/DPI) 400mcg (A) + 6 mcg (B) | 1 Dose | 2.77 | 2058(E) | 30.06.2017 |
| 121 | Bupivacaine | Injection 0.25% | 1 ml | 2.30 | 2058(E) | 30.06.2017 |
| 122 | Bupivacaine | Injection 0.5%with 7.5% glucose | 1 ml | 5.83 | 2058(E) | 30.06.2017 |
| 123 | Bupivacaine | Injection 0.50% | 1 ml | 3.68 | 2058(E) | 30.06.2017 |
| 124 | Caffeine | Injection 20 mg/ml | 1 ml | 235.59 | 2058(E) | 30.06.2017 |
| 125 | Caffeine | Oral Liquid 20mg/ml | 1 ml | 200.09 | 2058(E) | 30.06.2017 |
| 126 | Calamine | Lotion (As per IP) | 1 ml | 0.80 | 2058(E) | 30.06.2017 |
| 127 | Calcium folinate | Tablet 15 mg | 1 Tablet | 38.29 | 2059(E) | 30.06.2017 |
| 128 | Calcium gluconate | Injection 100mg/ml | 1 ml | 0.52 | 3725(E) | 23.11.2017 |
| 129 | Capecitabine | Tablet 500 mg | 1 Tablet | 118.62 | 2400(E) | 28.07.2017 |
| 130 | Capreomycin | Powder for Injection 1 gm | Each Pack | 325.11 | 2058(E) | 30.06.2017 |
| 131 | Carbamazepine | Oral Liquid 100mg/5ml | 1 ml | 0.18208 | 2058(E) | 30.06.2017 |
| 132 | Carbamazepine | CR Tablet 200 mg | 1 Tablet | 1.46 | 2058(E) | 30.06.2017 |
| 133 | Carbamazepine | CR Tablet 400 mg | 1 Tablet | 2.87 | 2058(E) | 30.06.2017 |
| 134 | Carbamazepine | Tablet 100 mg | 1 Tablet | 0.64 | 2058(E) | 30.06.2017 |
| 135 | Carbamazepine | Tablet 200 mg | 1 Tablet | 1.30 | 2058(E) | 30.06.2017 |
| 136 | Carbamazepine | Tablet 400 mg | 1 Tablet | 3.10 | 2058(E) | 30.06.2017 |
| 137 | Carbimazole | Tablet 10 mg | 1 Tablet | 3.47 | 2058(E) | 30.06.2017 |
| 138 | Carbimazole | Tablet 5 mg | 1 Tablet | 1.78 | 2058(E) | 30.06.2017 |
| 139 | Carboplatin | Injection 10 mg/ml | 1 ml | 49.95 | 2058(E) | 30.06.2017 |
| 140 | Carboxymethylcellulose | Drops 0.5% | 1 ml | 11.99 | 2058(E) | 30.06.2017 |
| 141 | Carboxymethylcellulose | Drops 1% | 1 ml | 16.62 | 2058(E) | 30.06.2017 |
| 142 | Cefadroxil | Capsule 500 mg | 1 Capsule | 6.71 | 2058(E) | 30.06.2017 |
| 143 | Cefadroxil | Oral Liquid 125 mg/5ml | 1 ml | 0.59 | 2058(E) | 30.06.2017 |
| 144 | Cefadroxil | Tablet 1 gm | 1 Tablet | 5.90 | 2058(E) | 30.06.2017 |
| 145 | Cefadroxil | Tablet 500 mg | 1 Tablet | 3.79 | 2058(E) | 30.06.2017 |
| 146 | Cefazolin | Powder for Injection 1 gm | Each Pack | 23.05 | 2058(E) | 30.06.2017 |
| 147 | Cefazolin | Powder for Injection 500 mg | Each Pack | 14.61 | 2058(E) | 30.06.2017 |
| 148 | Cefixime | Capsule 200 mg | 1 Capsule | 13.54 | 2058(E) | 30.06.2017 |
| 149 | Cefixime | Capsule 400 mg | 1 Capsule | 27.44 | 2058(E) | 30.06.2017 |
| 150 | Cefixime | Oral Liquid 100mg/5ml | 1 ml | 2.02 | 2058(E) | 30.06.2017 |
| 151 | Cefixime | Oral Liquid 50mg/5ml | 1 ml | 1.36 | 2058(E) | 30.06.2017 |
| 152 | Cefixime | Tablet 400 mg | 1 Tablet | 20.71 | 2058(E) | 30.06.2017 |
| 153 | Cefixime | Tablet 200mg | 1 Tablet | 9.01 | 2401(E) | 28.07.2017 |
| 154 | Cefotaxime | Powder forInjection 1 gm | Each Pack | 32.22 | 2058(E) | 30.06.2017 |
| 155 | Cefotaxime | Powder for Injection 250 mg | Each Pack | 14.65 | 2058(E) | 30.06.2017 |
| 156 | Cefotaxime | Powder forInjection 500 mg | Each Pack | 19.26 | 2058(E) | 30.06.2017 |
| 157 | Ceftazidime | Powder for Injection 1 gm | Each Pack | 196.44 | 2058(E) | 30.06.2017 |
| 158 | Ceftazidime | Powder forInjection 250 mg | Each Pack | 61.09 | 2058(E) | 30.06.2017 |
| 159 | Ceftriaxone | Powder for Injection 1gm | Each Pack | 50.60 | 3729(E) | 23.11.2017 |
| 160 | Ceftriaxone | Powder for | Each Pack | 120.28 | 2058(E) | 30.06.2017 |
| Injection 2 gm | ||||||
| 161 | Ceftriaxone | Powder for Injection 250 mg | Each Pack | 23.16 | 2058(E) | 30.06.2017 |
| 162 | Ceftriaxone | Powder forInjection 500 mg | Each Pack | 42.31 | 2058(E) | 30.06.2017 |
| 163 | Cetirizine | Capsule 10 mg | 1 Capsule | 2.23 | 2058(E) | 30.06.2017 |
| 164 | Cetirizine | Oral Liquid 5mg/5ml | 1 ml | 0.56 | 2058(E) | 30.06.2017 |
| 165 | Cetirizine | Tablet 10 mg | 1 Tablet | 1.55 | 2058(E) | 30.06.2017 |
| 166 | Cetrimide | Solution 20%(concentrate for dilution) | 1 ml | 0.30 | 2058(E) | 30.06.2017 |
| 167 | Chlorambucil | Tablet 2 mg | 1 Tablet | 34.64 | 2059(E) | 30.06.2017 |
| 168 | Chlorambucil | Tablet 5 mg | 1 Tablet | 77.85 | 2059(E) | 30.06.2017 |
| 169 | Chlorhexidine | Solution 5%(Concentrate for
dilution) |
1 ml | 0.14682 | 3090(E) | 20.09.2017 |
| 170 | Chloroquine | Oral Liquid 50mg/5ml | 1 ml | 0.28 | 2059(E) | 30.06.2017 |
| 171 | Chloroquine | Tablet 150mg | 1 Tablet | 0.74 | 3729(E) | 23.11.2017 |
| 172 | Chlorpheniramine | Tablet 4 mg | 1 Tablet | 0.08612 | 2058(E) | 30.06.2017 |
| 173 | Cholecalciferol | Capsule 1000 IU | 1 Capsule | 4.48 | 2058(E) | 30.06.2017 |
| 174 | Cholecalciferol | Capsule 60000 IU | 1 Capsule | 27.72 | 2058(E) | 30.06.2017 |
| 175 | Cholecalciferol | Oral Liquid 400 IU/ml | 1 ml | 2.21 | 2058(E) | 30.06.2017 |
| 176 | Cholecalciferol | Tablet 1000 IU | 1 Tablet | 3.52 | 2058(E) | 30.06.2017 |
| 177 | Cholecalciferol | Tablet 60000 IU | 1 Tablet | 22.76 | 2058(E) | 30.06.2017 |
| 178 | Ciprofloxacin | Ointment 0.3% | 1 gm | 1.06 | 2058(E) | 30.06.2017 |
| 179 | Ciprofloxacin | Drops 0.30% | 1 ml | 1.43 | 2058(E) | 30.06.2017 |
| 180 | Ciprofloxacin | Injection 200 mg/100ml | 1 ml | 0.15841 | 2058(E) | 30.06.2017 |
| 181 | Ciprofloxacin | Oral Liquid 250mg/5ml | 1 ml | 0.59 | 2058(E) | 30.06.2017 |
| 182 | Ciprofloxacin | Tablet 250mg | 1 Tablet | 1.95 | 2401(E) | 28.07.2017 |
| 183 | Ciprofloxacin | Tablet 500mg | 1 Tablet | 3.42 | 2401(E) | 28.07.2017 |
| 184 | Cisplatin | Injection 1mg/ml | 1 ml | 6.46 | 2058(E) | 30.06.2017 |
| 185 | Clarithromycin | Oral Liquid 125mg/5ml | 1 ml | 4.05 | 2058(E) | 30.06.2017 |
| 186 | Clarithromycin | Tablet 250 mg | 1 Tablet | 26.28 | 2058(E) | 30.06.2017 |
| 187 | Clarithromycin | Tablet 500 mg | 1 Tablet | 46.32 | 2058(E) | 30.06.2017 |
| 188 | Clindamycin | Capsule 150 mg | 1 Capsule | 13.58 | 2059(E) | 30.06.2017 |
| 189 | Clindamycin | Capsule 300 mg | 1 Capsule | 22.17 | 2059(E) | 30.06.2017 |
| 190 | Clindamycin | Tablet 300 mg | 1 Tablet | 14.31 | 2059(E) | 30.06.2017 |
| 191 | Clobazam | Tablet 10 mg | 1 Tablet | 8.48 | 2058(E) | 30.06.2017 |
| 192 | Clobazam | Tablet 5 mg | 1 Tablet | 4.82 | 2058(E) | 30.06.2017 |
| 193 | Clofazimine | Capsule 100 mg | 1 Capsule | 2.32 | 2059(E) | 30.06.2017 |
| 194 | Clofazimine | Capsule 50 mg | 1 Capsule | 1.36 | 2059(E) | 30.06.2017 |
| 195 | Clomiphene | Capsule 100 mg | 1 Capsule | 52.24 | 2058(E) | 30.06.2017 |
| 196 | Clomiphene | Capsule 50 mg | 1 Capsule | 28.74 | 2058(E) | 30.06.2017 |
| 197 | Clomiphene | Tablet 100mg | 1 Tablet | 11.46 | 650(E) | 13.02.2018 |
| 198 | Clomiphene | Tablet 50mg | 1 Tablet | 7.82 | 650(E) | 13.02.2018 |
| 199 | Clomipramine | Tablet 10 mg | 1 Tablet | 2.36 | 2058(E) | 30.06.2017 |
| 200 | Clomipramine | Tablet 25 mg | 1 Tablet | 5.21 | 2058(E) | 30.06.2017 |
| 201 | Clomipramine | Tablet 75 mg | 1 Tablet | 13.47 | 2058(E) | 30.06.2017 |
| 202 | Clonazepam | Tablet 0.25 mg | 1 Tablet | 1.72 | 2058(E) | 30.06.2017 |
| 203 | Clonazepam | Tablet 0.5 mg | 1 Tablet | 2.92 | 2058(E) | 30.06.2017 |
| 204 | Clonazepam | Tablet 1 mg | 1 Tablet | 3.97 | 2058(E) | 30.06.2017 |
| 205 | Clopidogrel | Tablet 75 mg | 1 Tablet | 6.52 | 2058(E) | 30.06.2017 |
| 206 | Clotrimazole | Cream 1% | 1 gm | 2.54 | 2058(E) | 30.06.2017 |
| 207 | Clotrimazole | Drops 1% | 1 ml | 2.84 | 2058(E) | 30.06.2017 |
| 208 | Clotrimazole | Lotion 1% | 1 ml | 3.36 | 2058(E) | 30.06.2017 |
| 209 | Clotrimazole | Pessary 100 mg | 1 Pessary | 8.15 | 2058(E) | 30.06.2017 |
| 210 | Cloxacillin | Capsule 250mg | 1 Capsule | 1.06 | 2602(E) | 11.08.2017 |
| 211 | Cloxacillin | Capsule 500mg | 1 Capsule | 1.78 | 2602(E) | 11.08.2017 |
| 212 | Cloxacillin | Tablet 250mg | 1 Tablet | 1.62 | 2601(E) | 11.08.2017 |
| 213 | Cloxacillin | Powder forInjection250mg | Each Pack | 4.17 | 3090(E) | 20.09.2017 |
| 214 | Clozapine | Tablet 100 mg | 1 Tablet | 7.27 | 2058(E) | 30.06.2017 |
| 215 | Clozapine | Tablet 25 mg | 1 Tablet | 2.36 | 2058(E) | 30.06.2017 |
| 216 | Clozapine | Tablet 50 mg | 1 Tablet | 4.51 | 2058(E) | 30.06.2017 |
| 217 | Coagulation factor IX | Powder for Injection 600 IU | Each Pack | 11,564.38 | 3725(E) | 23.11.2017 |
| 218 | Coagulation factor VIII | Powder forInjection 250 IU | Each pack | 3,505.66 | 2400(E) | 28.07.2017 |
| 219 | Coagulation factor VIII | Powder for Injection 500 IU | Each Pack | 8,602.64 | 2400(E) | 28.07.2017 |
| 220 | Colchicine | Tablet 0.5 mg | 1 Tablet | 2.92 | 2058(E) | 30.06.2017 |
| 221 | Co-trimoxazole (Sulphamethoxazole (A)+Trimethoprim (B)] | Oral Liquid 200 mg(A)+40mg(B)/5ml | 1 ml | 0.20231 | 2058(E) | 30.06.2017 |
| 222 | Co-trimoxazole(Sulphamethoxazole (A)+Trimethoprim (B)] | Tablet 400 mg(A)+80 mg(B) | 1 Tablet | 0.49 | 2058(E) | 30.06.2017 |
| 223 | Co-trimoxazole(Sulphamethoxazole
(A)+Trimethoprim (B)] |
Tablet 800mg(A)+160
mg(B) |
1 Tablet | 0.99 | 2058(E) | 30.06.2017 |
| 224 | Cyclophosphamide | Tablet 50 mg | 1 Tablet | 3.81 | 2059(E) | 30.06.2017 |
| 225 | Cyclophosphamide | Powder for Injection 500 mg | Each Pack | 74.91 | 2059(E) | 30.06.2017 |
| 226 | Cycloserine | Capsule 250 mg | 1 Capsule | 50.96 | 2058(E) | 30.06.2017 |
| 227 | Cycloserine | Tablet 250 mg | 1 Tablet | 43.81 | 2058(E) | 30.06.2017 |
| 228 | Cyclosporine | Capsule 100 mg | 1 Capsule | 96.21 | 2059(E) | 30.06.2017 |
| 229 | Cyclosporine | Capsule 25 mg | 1 Capsule | 25.82 | 2059(E) | 30.06.2017 |
| 230 | Cyclosporine | Capsule 50 mg | 1 Capsule | 49.41 | 2059(E) | 30.06.2017 |
| 231 | Cyclosporine | Injection 50 mg/ml | 1 ml | 253.16 | 2059(E) | 30.06.2017 |
| 232 | Cyclosporine | Oral Liquid 100mg/ml | 1 ml | 88.72 | 2059(E) | 30.06.2017 |
| 233 | Cytosine arabinoside | Injection 100 mg/ml | Each Pack | 186.47 | 2059(E) | 30.06.2017 |
| 234 | Cytosine arabinoside | Powder for | Each Pack | 1,005.74 | 2059(E) | 30.06.2017 |
| Injection 1000 mg | ||||||
| 235 | Cytosine arabinoside | Powder forInjection 500 mg | Each Pack | 480.69 | 2059(E) | 30.06.2017 |
| 236 | Dacarbazine | Powder for Injection 200 mg | Each Pack | 412.34 | 2058(E) | 30.06.2017 |
| 237 | Dacarbazine | Powder forInjection 500 mg | Each Pack | 930.19 | 2058(E) | 30.06.2017 |
| 238 | Dapsone | Tablet 100 mg | 1 Tablet | 0.22150 | 2059(E) | 30.06.2017 |
| 239 | Darunavir | Tablet 600 mg | 1 Tablet | 156.67 | 2058(E) | 30.06.2017 |
| 240 | Desferrioxamine | Powder for Injection 500mg | Each pack | 150.93 | 3723(E) | 23.11.2017 |
| 241 | Dexamethasone | Tablet 0.5 mg | 1 Tablet | 0.18208 | 2058(E) | 30.06.2017 |
| 242 | Dexamethasone | Injection 4 mg/ml | Each Pack (10 ml) | 12.93 | 2058(E) | 30.06.2017 |
| 243 | Dexamethasone | Injection 4 mg/ml | Each Pack (2 ml) | 5.34 | 2058(E) | 30.06.2017 |
| 244 | Dexamethasone | Injection 4mg/ml | Each Pack (20 ml) | 24.77 | 2058(E) | 30.06.2017 |
| 245 | Dexamethasone | Injection 4 mg/ml | Each Pack (30 ml) | 33.11 | 2058(E) | 30.06.2017 |
| 246 | Dextran-40 | Injection 10% | 1 ml | 0.39 | 3725(E) | 23.11.2017 |
| 247 | Diazepam | Injection 5mg/ml | 1 ml | 5.69 | 2058(E) | 30.06.2017 |
| 248 | Diazepam | Oral Liquid 2 mg/5ml | 1 ml | 2.57 | 2058(E) | 30.06.2017 |
| 249 | Diazepam | Suppository 5 mg | 1 Suppository | 5.61 | 2058(E) | 30.06.2017 |
| 250 | Diazepam | Tablet 2 mg | 1 Tablet | 1.41 | 2058(E) | 30.06.2017 |
| 251 | Diazepam | Tablet 5 mg | 1 Tablet | 1.33 | 2058(E) | 30.06.2017 |
| 252 | Diclofenac | Capsule 50 mg | 1 Capsule | 0.58 | 2058(E) | 30.06.2017 |
| 253 | Diclofenac | Injection 25 mg/ml | 1 ml | 1.37 | 2058(E) | 30.06.2017 |
| 254 | Diclofenac | Tablet 50 mg | 1 Tablet | 1.79 | 2058(E) | 30.06.2017 |
| 255 | Dicyclomine | Injection 10mg/ml (10ml & 30ml Pack) | 1 ml | 1.93 | 2058(E) | 30.06.2017 |
| 256 | Dicyclomine | Injection 10 mg/ml (1ml &2ml Pack) | 1 ml | 2.99 | 2058(E) | 30.06.2017 |
| 257 | Dicyclomine | Oral Solution 10mg/5ml | 1 ml | 0.15608 | 3091(E) | 20.09.2017 |
| 258 | Dicyclomine | Tablet 10mg | 1 Tablet | 0.10297 | 3090(E) | 20.09.2017 |
| 259 | Diethylcarbamazine | Oral Liquid 120 mg/5ml | 1 ml | 0.46 | 2059(E) | 30.06.2017 |
| 260 | Diethylcarbamazine | Tablet 100 mg | 1 Tablet | 1.32 | 2059(E) | 30.06.2017 |
| 261 | Diethylcarbamazine | Tablet 50 mg | 1 Tablet | 0.53 | 2059(E) | 30.06.2017 |
| 262 | Digoxin | Injection 0.25 mg/ml | 1 ml | 3.13 | 2058(E) | 30.06.2017 |
| 263 | Digoxin | Oral liquid 0.05mg/ml | 1 ml | 0.30 | 3725(E) | 23.11.2017 |
| 264 | Digoxin | Tablet 0.25 mg | 1 Tablet | 1.12 | 2058(E) | 30.06.2017 |
| 265 | Diloxanide furoate | Tablet 500mg | 1 Tablet | 0.99 | 3090(E) | 20.09.2017 |
| 266 | Diltiazem | Capsule 90 mg | 1 Capsule | 9.02 | 2058(E) | 30.06.2017 |
| 267 | Diltiazem | Injection 5mg/ml | 1 ml | 2.96 | 2058(E) | 30.06.2017 |
| 268 | Diltiazem | SR Tablet 90 mg | 1 Tablet | 8.76 | 2058(E) | 30.06.2017 |
| 269 | Diltiazem | Tablet 30 mg | 1 Tablet | 2.25 | 2058(E) | 30.06.2017 |
| 270 | Diltiazem | Tablet 60 mg | 1 Tablet | 4.58 | 2058(E) | 30.06.2017 |
| 271 | Dinoprostone | Gel 0.5 mg | 1 gm | 76.08 | 2058(E) | 30.06.2017 |
| 272 | Dinoprostone | Tablet 0.5mg | 1 Tablet | 46.98 | 2397(E) | 28.07.2017 |
| 273 | Diphtheria antitoxin | 10000 IU | Each Pack | 1,230.11 | 3090(E) | 20.09.2017 |
| 274 | Dobutamine | Injection 50 mg/ml | Each Pack | 35.20 | 2058(E) | 30.06.2017 |
| 275 | Docetaxel | Powder for Injection 20 mg | Each Pack | 2,809.52 | 2058(E) | 30.06.2017 |
| 276 | Docetaxel | Powder forInjection 80 mg | Each Pack | 10,681.99 | 2058(E) | 30.06.2017 |
| 277 | Domperidone | Oral Liquid 1 mg/ml | 1 ml | 1.01 | 2058(E) | 30.06.2017 |
| 278 | Domperidone | Tablet 10 mg | 1 Tablet | 2.27 | 2058(E) | 30.06.2017 |
| 279 | Donepezil | Tablet 10 mg | 1 Tablet | 15.06 | 2058(E) | 30.06.2017 |
| 280 | Donepezil | Tablet 5 mg | 1 Tablet | 10.55 | 2058(E) | 30.06.2017 |
| 281 | Dopamine | Injection 40 mg/ml | 1 ml | 5.30 | 2059(E) | 30.06.2017 |
| 282 | Doxorubicin | Injection 2 mg/ml | 1 ml | 33.70 | 2058(E) | 30.06.2017 |
| 283 | Doxycycline | Capsule 100 mg | 1 Capsule | 2.35 | 2058(E) | 30.06.2017 |
| 284 | Doxycycline | Tablet 100 mg | 1 Tablet | 0.91 | 2058(E) | 30.06.2017 |
| 285 | DPT + Hib + Hep B vaccine | Per 0.1 ml | 75.38 | 2059(E) | 30.06.2017 | |
| 286 | DPT vaccine | Per 0.5 ml | 13.29 | 2059(E) | 30.06.2017 | |
| 287 | Efavirenz | Capsule 200 mg | 1 Capsule | 22.60 | 2059(E) | 30.06.2017 |
| 288 | Efavirenz | Capsule 600 mg | 1 Capsule | 58.87 | 2059(E) | 30.06.2017 |
| 289 | Efavirenz | Tablet 200 mg | 1 Tablet | 21.28 | 2059(E) | 30.06.2017 |
| 290 | Efavirenz | Tablet 600 mg | 1 Tablet | 66.66 | 2059(E) | 30.06.2017 |
| 291 | Enalapril | Tablet 2.5 mg | 1 Tablet | 1.81 | 2058(E) | 30.06.2017 |
| 292 | Enalapril | Tablet 5 mg | 1 Tablet | 3.01 | 2058(E) | 30.06.2017 |
| 293 | Enoxaparin | Injection 40mg/0.4ml | 0.1 ml | 95.41 | 2059(E) | 30.06.2017 |
| 294 | Enoxaparin | Injection 60 mg/0.6ml | 0.1 ml | 95.41 | 2059(E) | 30.06.2017 |
| 295 | Entecavir | Tablet 0.5 mg | 1 Tablet | 74.89 | 2059(E) | 30.06.2017 |
| 296 | Entecavir | Tablet 1 mg | 1 Tablet | 118.98 | 2059(E) | 30.06.2017 |
| 297 | Erythropoietin | Injection 10000 IU/ml | Each pack | 2,441.72 | 2058(E) | 30.06.2017 |
| 298 | Erythropoietin | Injection 2000IU/ml | Each pack | 578.33 | 2058(E) | 30.06.2017 |
| 299 | Escitalopram | Tablet 10 mg | 1 Tablet | 7.61 | 2058(E) | 30.06.2017 |
| 300 | Escitalopram | Tablet 20 mg | 1 Tablet | 12.01 | 2058(E) | 30.06.2017 |
| 301 | Escitalopram | Tablet 5 mg | 1 Tablet | 4.29 | 2058(E) | 30.06.2017 |
| 302 | Esmolol | Injection 10mg/ml | 1 ml | 20.33 | 2398(E) | 28.07.2017 |
| 303 | Ethambutol | Tablet 200 mg | 1 Tablet | 0.99 | 2400(E) | 28.07.2017 |
| 304 | Ethambutol | Tablet 400 mg | 1 Tablet | 2.25 | 2400(E) | 28.07.2017 |
| 305 | Ethambutol | Tablet 600 mg | 1 Tablet | 3.32 | 2400(E) | 28.07.2017 |
| 306 | Ethambutol | Tablet 800 mg | 1 Tablet | 3.97 | 2400(E) | 28.07.2017 |
| 307 | Ethinylestradiol | Tablet 0.01 mg | 1 Tablet | 2.21 | 2059(E) | 30.06.2017 |
| 308 | Ethinylestradiol | Tablet 0.05 mg | 1 Tablet | 3.52 | 2059(E) | 30.06.2017 |
| 309 | Ethinylestradiol (A) + Levonorgestrel (B) | Tablet 0.03mg(A) + 0.15
mg(B) |
1 Tablet | 3.24 | 2059(E) | 30.06.2017 |
| 310 | Ethionamide | Tablet 250 mg | 1 Tablet | 14.82 | 2058(E) | 30.06.2017 |
| 311 | Etoposide | Capsule 50 mg | 1 Capsule | 51.03 | 2058(E) | 30.06.2017 |
| 312 | Etoposide | Injection 20mg/ml | 1 ml | 33.65 | 2058(E) | 30.06.2017 |
| 313 | Fentanyl | Injection50mcg/ml | 1 ml | 21.31 | 2058(E) | 30.06.2017 |
| 314 | Ferrous salt (A) + Folic acid(B) | Oral liqiud 20mgelemental iron
(A) + 100mcg (B)/ml |
1 ml | 0.12489 | 3091(E) | 20.09.2017 |
| 315 | Ferrous salt (A) + Folic acid (B) | Tablet 100 mg elemental iron(A) + 500 mcg (B) | 1 Tablet | 0.10159 | 3091(E) | 20.09.2017 |
| 316 | Ferrous salt (A) + Folic acid(B) | Tablet45mgelemental
iron (A) + 400 mcg (B) |
1 Tablet | 0.13194 | 3091(E) | 20.09.2017 |
| 317 | Filgrastim | Injection 300mcg | Each pack | 1,288.03 | 2058(E) | 30.06.2017 |
| 318 | Fluconazole | Capsule 100 mg | 1 Capsule | 23.71 | 2058(E) | 30.06.2017 |
| 319 | Fluconazole | Capsule 150 mg | 1 Capsule | 17.41 | 2058(E) | 30.06.2017 |
| 320 | Fluconazole | Capsule 200 mg | 1 Capsule | 34.27 | 2058(E) | 30.06.2017 |
| 321 | Fluconazole | Oral Liquid 50mg/5ml | 1 ml | 2.46 | 3088(E) | 11.08.2017 |
| 322 | Fluconazole | Tablet 100 mg | 1 Tablet | 7.49 | 2058(E) | 30.06.2017 |
| 323 | Fluconazole | Tablet 150 mg | 1 Tablet | 11.12 | 2058(E) | 30.06.2017 |
| 324 | Fluconazole | Tablet 200 mg | 1 Tablet | 17.63 | 2058(E) | 30.06.2017 |
| 325 | Fluconazole | Tablet 400 mg | 1 Tablet | 29.32 | 2058(E) | 30.06.2017 |
| 326 | Fluconazole | Injection 200 mg/100 ml | Each Pack | 99.89 | 2058(E) | 30.06.2017 |
| 327 | Flunarizine | Tablet 10 mg | 1 Tablet | 4.44 | 2058(E) | 30.06.2017 |
| 328 | Flunarizine | Tablet 5 mg | 1 Tablet | 2.59 | 2058(E) | 30.06.2017 |
| 329 | Fluoxetine | Capsule 10 mg | 1 Capsule | 2.79 | 2058(E) | 30.06.2017 |
| 330 | Fluoxetine | Capsule 20 mg | 1 Capsule | 3.42 | 2058(E) | 30.06.2017 |
| 331 | Fluoxetine | Capsule 40 mg | 1 Capsule | 5.20 | 2058(E) | 30.06.2017 |
| 332 | Fluoxetine | Capsule 60 mg | 1 Capsule | 9.19 | 2058(E) | 30.06.2017 |
| 333 | Fluoxetine | Tablet 10 mg | 1 Tablet | 2.34 | 2058(E) | 30.06.2017 |
| 334 | Fluoxetine | Tablet 20 mg | 1 Tablet | 3.72 | 2058(E) | 30.06.2017 |
| 335 | Fluoxetine | Tablet 40 mg | 1 Tablet | 5.01 | 2058(E) | 30.06.2017 |
| 336 | Fluoxetine | Tablet 60 mg | 1 Tablet | 6.94 | 2058(E) | 30.06.2017 |
| 337 | Fluphenazine | Depot Injection 25 mg/ml | 1 ml | 46.96 | 2058(E) | 30.06.2017 |
| 338 | Folic Acid | Capsule 5 mg | 1 Capsule | 5.29 | 2058(E) | 30.06.2017 |
| 339 | Folic Acid | Tablet 5 mg | 1 Tablet | 1.30 | 2058(E) | 30.06.2017 |
| 340 | Furosemide | Oral liquid 10mg/ml | 1 ml | 0.30 | 3724(E) | 23.11.2017 |
| 341 | Furosemide | Tablet 40 mg | 1 Tablet | 0.47 | 2058(E) | 30.06.2017 |
| 342 | Furosemide | Injection 10mg/ml | 1 ml | 1.55 | 884(E) | 28.02.2018 |
| 343 | Fusidic acid | Cream 2% | 1 gm | 8.56 | 2058(E) | 30.06.2017 |
| 344 | Gadobenate | Injection 529mg/ml | 1 ml | 102.30 | 3724(E) | 23.11.2017 |
| 345 | Ganciclovir | Capsule 250 mg | 1 Capsule | 112.84 | 2059(E) | 30.06.2017 |
| 346 | Ganciclovir | Powder for Injection 500 mg | Each Pack | 1,583.95 | 2059(E) | 30.06.2017 |
| 347 | Gefitinib | Tablet 250 mg | 1 Tablet | 402.28 | 2058(E) | 30.06.2017 |
| 348 | Gemcitabine | Powder for Injection 1 gm | Each Pack | 4,979.45 | 2058(E) | 30.06.2017 |
| 349 | Gemcitabine | Powder forInjection 200mg | Each Pack | 1,099.70 | 269(E) | 16.01.2018 |
| 350 | Gentamicin | Drops 0.3% | 1 ml | 0.85 | 2058(E) | 30.06.2017 |
| 351 | Gentamicin | Injection 10mg/ml | 1 ml | 2.98 | 2058(E) | 30.06.2017 |
| 352 | Gentamicin | Injection 40mg/ml | Each Pack (10ml) | 16.86 | 2058(E) | 30.06.2017 |
| 353 | Gentamicin | Injection 40 mg/ml | Each Pack (2 ml) | 4.52 | 2058(E) | 30.06.2017 |
| 354 | Gentamicin | Injection 40mg/ml | Each Pack (20 ml) | 25.56 | 2058(E) | 30.06.2017 |
| 355 | Gentamicin | Injection 40 mg/ml | Each Pack (30 ml) | 27.98 | 2058(E) | 30.06.2017 |
| 356 | Glimepiride | Tablet 1 mg | 1 Tablet | 3.37 | 2058(E) | 30.06.2017 |
| 357 | Glimepiride | Tablet 2 mg | 1 Tablet | 5.35 | 2058(E) | 30.06.2017 |
| 358 | Glucose | Injection 25% | 1 ml | 0.16138 | 2058(E) | 30.06.2017 |
| 359 | Glucose | Injection 50% | 1 ml | 0.46 | 3724(E) | 23.11.2017 |
| 360 | Glucose | Injection 5% | 1000ml Glass | 60.29 | 2058(E) | 30.06.2017 |
| 361 | Glucose | Injection 5% | 1000ml Non-Glass | 48.65 | 2058(E) | 30.06.2017 |
| 362 | Glucose | Injection 5% | 100ml Glass | 16.92 | 2058(E) | 30.06.2017 |
| 363 | Glucose | Injection 5% | 100ml Non-Glass | 15.32 | 2058(E) | 30.06.2017 |
| 364 | Glucose | Injection 5% | 250ml Glass | 23.38 | 2058(E) | 30.06.2017 |
| 365 | Glucose | Injection 5% | 250ml Non-Glass | 21.12 | 2058(E) | 30.06.2017 |
| 366 | Glucose | Injection 5% | 500ml Glass | 33.18 | 2058(E) | 30.06.2017 |
| 367 | Glucose | Injection 5% | 500ml Non-Glass | 28.64 | 2058(E) | 30.06.2017 |
| 368 | Glucose | Injection 10% | Each Pack(1000 ml) | 24.24 | 2058(E) | 30.06.2017 |
| 369 | Glucose | Injection 10% | Each Pack(500 ml) | 26.37 | 2058(E) | 30.06.2017 |
| 370 | Glucose (A) + Sodium Chloride (B) | Injection 5% (A)+ 0.9% (B) | 1000ml Glass | 60.67 | 2058(E) | 30.06.2017 |
| 371 | Glucose (A) + Sodium Chloride (B) | Injection 5% (A)+ 0.9% (B) | 1000ml Non- Glass | 49.03 | 2058(E) | 30.06.2017 |
| 372 | Glucose (A) + Sodium Chloride | Injection 5% (A) | 100ml Glass | 16.97 | 2058(E) | 30.06.2017 |
| (B) | + 0.9% (B) | |||||
| 373 | Glucose (A) + Sodium Chloride (B) | Injection 5% (A)+ 0.9% (B) | 100ml Non- Glass | 15.34 | 2058(E) | 30.06.2017 |
| 374 | Glucose (A) + Sodium Chloride(B) | Injection 5% (A)+ 0.9% (B) | 250ml Glass | 23.48 | 2058(E) | 30.06.2017 |
| 375 | Glucose (A) + Sodium Chloride (B) | Injection 5% (A)+ 0.9% (B) | 250ml Non- Glass | 21.20 | 2058(E) | 30.06.2017 |
| 376 | Glucose (A) + Sodium Chloride (B) | Injection 5% (A)+ 0.9% (B) | 500ml Glass | 33.35 | 2058(E) | 30.06.2017 |
| 377 | Glucose (A) + Sodium Chloride(B) | Injection 5% (A)+ 0.9% (B) | 500ml Non- Glass | 28.81 | 2058(E) | 30.06.2017 |
| 378 | Glutaraldehyde | Solution 2% | 1 ml | 0.06070 | 3723(E) | 23.11.2017 |
| 379 | Glycerin | Oral Liquid | 1 ml | 0.14067 | 3090(E) | 20.09.2017 |
| 380 | Glyceryl Trinitrate | Injection 5 mg/ml | 1 ml | 6.16 | 2058(E) | 30.06.2017 |
| 381 | Glyceryl Trinitrate | Sublingual Tablet0.5 mg | 1 Tablet | 1.70 | 2058(E) | 30.06.2017 |
| 382 | Glycopyrrolate | Injection 0.2 mg/ml | 1 ml | 11.53 | 2058(E) | 30.06.2017 |
| 383 | Griseofulvin | Tablet 125mg | 1 Tablet | 0.77 | 3090(E) | 20.09.2017 |
| 384 | Griseofulvin | Tablet 250 mg | 1 Tablet | 1.50 | 2058(E) | 30.06.2017 |
| 385 | Griseofulvin | Tablet 375 mg | 1 Tablet | 4.04 | 2058(E) | 30.06.2017 |
| 386 | Haemodialysis fluid | As license | 1 ml | 0.03822 | 3724(E) | 23.11.2017 |
| 387 | Haloperidol | Injection 5mg/ml | 1 ml | 5.47 | 2058(E) | 30.06.2017 |
| 388 | Haloperidol | Oral Liquid 2mg/ 5ml | 1 ml | 1.93 | 2058(E) | 30.06.2017 |
| 389 | Haloperidol | Tablet 1.5 mg | 1 Tablet | 1.55 | 2058(E) | 30.06.2017 |
| 390 | Haloperidol | Tablet 10 mg | 1 Tablet | 4.14 | 2058(E) | 30.06.2017 |
| 391 | Haloperidol | Tablet 20 mg | 1 Tablet | 4.62 | 2058(E) | 30.06.2017 |
| 392 | Haloperidol | Tablet 5 mg | 1 Tablet | 3.23 | 2058(E) | 30.06.2017 |
| 393 | Halothane | Inhalation | 1 ml | 5.50 | 2058(E) | 30.06.2017 |
| 394 | Heparin | Injection 1000IU/ml | 1 ml | 15.31 | 2059(E) | 30.06.2017 |
| 395 | Heparin | Injection 5000IU/ml | 1 ml | 37.99 | 2059(E) | 30.06.2017 |
| 396 | Hepatitis B immunoglobulin | Each Pack | 5,170.04 | 3088(E) | 11.08.2017 | |
| 397 | Hepatitis B vaccine | 1 ml | 73.50 | 2059(E) | 30.06.2017 | |
| 398 | Homatropine | Drops 2% | 1 ml | 5.83 | 2400(E) | 28.07.2017 |
| 399 | Hormone releasing IUD | Contains 52 mgofLevonorgestrel | 1 IUD | 3,630.63 | 2059(E) | 30.06.2017 |
| 400 | Human chorionicGonadotropin | Injection 1000 IU | Each Pack | 199.47 | 2059(E) | 30.06.2017 |
| 401 | Human chorionicGonadotropin | Injection 5000 IU | Each Pack | 391.73 | 2059(E) | 30.06.2017 |
| 402 | Human NormalImmunoglobulin | 1 ml | 144.79 | 2400(E) | 28.07.2017 | |
| 403 | Hydrochlorothiazide | Tablet 12.5 mg | 1 Tablet | 0.92 | 2058(E) | 30.06.2017 |
| 404 | Hydrochlorothiazide | Tablet 25 mg | 1 Tablet | 1.58 | 2058(E) | 30.06.2017 |
| 405 | Hydrochlorothiazide | Tablet 50 mg | 1 Tablet | 0.07767 | 2058(E) | 30.06.2017 |
| 406 | Hydrocortisone | Tablet 10 mg | 1 Tablet | 5.52 | 2059(E) | 30.06.2017 |
| 407 | Hydrocortisone | Tablet 5 mg | 1 Tablet | 2.84 | 2059(E) | 30.06.2017 |
| 408 | Hydrocortisone | Injection 100 mg | Each Pack | 36.52 | 2059(E) | 30.06.2017 |
| 409 | Hydrocortisone | Injection 200 mg | Each Pack | 56.31 | 2059(E) | 30.06.2017 |
| 410 | Hydrocortisone | Powder forInjection 100 mg | Each Pack | 36.52 | 2059(E) | 30.06.2017 |
| 411 | Hydrogen peroxide | Solution 6% | 1 ml | 0.03759 | 3090(E) | 20.09.2017 |
| 412 | Hydroxychloroquine | Tablet 200 mg | 1 Tablet | 5.56 | 2058(E) | 30.06.2017 |
| 413 | Hydroxychloroquine | Tablet 400 mg | 1 Tablet | 11.21 | 2058(E) | 30.06.2017 |
| 414 | Hydroxypropyl methylcellulose | Injection 2% | 1 ml | 35.20 | 2058(E) | 30.06.2017 |
| 415 | Hydroxyurea | Capsule 500 mg | 1 Capsule | 12.03 | 2059(E) | 30.06.2017 |
| 416 | Hyoscine butylbromide | Injection 20 mg/ml | 1 ml | 9.79 | 2058(E) | 30.06.2017 |
| 417 | Hyoscine Butylbromide | Tablet 10 mg | 1 Tablet | 2.79 | 2058(E) | 30.06.2017 |
| 418 | Ibuprofen | Capsule 400 mg | 1 Capsule | 0.99 | 2058(E) | 30.06.2017 |
| 419 | Ibuprofen | Tablet 200 mg | 1 Tablet | 0.36 | 2058(E) | 30.06.2017 |
| 420 | Ibuprofen | Tablet 400 mg | 1 Tablet | 0.65 | 2058(E) | 30.06.2017 |
| 421 | Ibuprofen | Oral Liquid 100mg/5ml | 1 ml | 0.18552 | 2603(E) | 11.08.2017 |
| 422 | Ifosfamide | Powder forInjection 1g | Each Pack | 339.62 | 3089(E) | 20.09.2017 |
| 423 | Ifosfamide | Powder forInjection 2g | Each Pack | 891.46 | 2601(E) | 11.08.2017 |
| 424 | Imatinib | Capsule 100 mg | 1 Capsule | 72.86 | 2058(E) | 30.06.2017 |
| 425 | Imatinib | Capsule 400 mg | 1 Capsule | 239.73 | 2058(E) | 30.06.2017 |
| 426 | Imatinib | Tablet 100 mg | 1 Tablet | 74.55 | 2058(E) | 30.06.2017 |
| 427 | Imatinib | Tablet 400 mg | 1 Tablet | 215.79 | 2058(E) | 30.06.2017 |
| 428 | Insulin (Soluble) | Injection 40 IU/ml | 1 ml | 14.13 | 2059(E) | 30.06.2017 |
| 429 | Intermediate Acting (NPH)Insulin | Injection 40IU/ml | 1 ml | 14.13 | 2059(E) | 30.06.2017 |
| 430 | Iohexol | Injection 300mgiodine/ml | 1 ml | 15.60 | 3088(E) | 11.08.2017 |
| 431 | Ipratropium | Inhalation (MDI/DPI) 20mcg/dose | 1 Dose | 0.58 | 2058(E) | 30.06.2017 |
| 432 | Ipratropium | Respirator solution for use in Nebulizer 250mcg/ml | 1 ml | 2.38 | 2058(E) | 30.06.2017 |
| 433 | Iron sucrose | Injection 20mg/ml | 1 ml | 50.45 | 2058(E) | 30.06.2017 |
| 434 | Isoflurane | Inhalation | 1 ml | 9.81 | 2059(E) | 30.06.2017 |
| 435 | Isoniazid | Tablet 100mg | 1 Tablet | 0.64 | 3089(E) | 20.09.2017 |
| 436 | Isoniazid | Tablet 300mg | 1 Tablet | 1.12 | 3088(E) | 11.08.2017 |
| 437 | Isosorbide 5 Mononitrate | Tablet 20 mg | 1 Tablet | 3.08 | 650(E) | 13.02.2018 |
| 438 | Isosorbide dinitrate | Tablet 10 mg | 1 Tablet | 0.69 | 2058(E) | 30.06.2017 |
| 439 | Isosorbide dinitrate | Tablet 5 mg | 1 Tablet | 0.71 | 2058(E) | 30.06.2017 |
| 440 | Isosorbide-5-mononitrate | SR Capsule 30 mg | 1 Capsule | 3.29 | 2058(E) | 30.06.2017 |
| 441 | Isosorbide-5-mononitrate | SR Capsule 60 mg | 1 Capsule | 3.27 | 2058(E) | 30.06.2017 |
| 442 | Isosorbide-5-mononitrate | SR Tablet 30 mg | 1 Tablet | 4.59 | 2058(E) | 30.06.2017 |
| 443 | Isosorbide-5-mononitrate | SR Tablet 60 mg | 1 Tablet | 5.99 | 2058(E) | 30.06.2017 |
| 444 | Isosorbide-5-mononitrate | Tablet 10 mg | 1 Tablet | 1.84 | 2058(E) | 30.06.2017 |
| 445 | Ispaghula | Granules/ Husk/ Powder | 1 gm | 0.73 | 2058(E) | 30.06.2017 |
| 446 | IUD containing Copper | As licensed | 1 IUD | 269.84 | 2059(E) | 30.06.2017 |
| 447 | Japanese Encephalitis Vaccine | 4mcg to 6mcg | Each Pack | 654.71 | 3722(E) | 23.11.2017 |
| 448 | Japanese Encephalitis Vaccine | up to 3mcg | Each Pack | 498.80 | 3722(E) | 23.11.2017 |
| 449 | Kanamycin | Powder for Injection 1 gm | Each Pack | 36.70 | 2058(E) | 30.06.2017 |
| 450 | Kanamycin | Powder forInjection 500 mg | Each Pack | 20.03 | 2058(E) | 30.06.2017 |
| 451 | Kanamycin | Powder for Injection 750 mg | Each Pack | 34.21 | 2058(E) | 30.06.2017 |
| 452 | Ketamine | Injection 10mg/ml | 1 ml | 9.94 | 2058(E) | 30.06.2017 |
| 453 | Ketamine | Injection 50 mg/ml | 1 ml | 9.81 | 2058(E) | 30.06.2017 |
| 454 | Labetalol | Injection 5mg/ml | 1 ml | 47.58 | 2058(E) | 30.06.2017 |
| 455 | Lactulose | Oral Liquid 10 g/15 ml | 1 ml | 1.03 | 2059(E) | 30.06.2017 |
| 456 | Lamivudine (A) + Nevirapine(B) + Stavudine (C) | Tablet 150 mg(A)+ 200 mg(B) + 30
mg(C) |
1 Tablet | 14.65 | 2400(E) | 28.07.2017 |
| 457 | Lamivudine (A) + Zidovudine(B) | Tablet 150 mg(A)+ 300 mg(B) | 1 Tablet | 18.83 | 2400(E) | 28.07.2017 |
| 458 | Lamivudine (A)+ Nevirapine(B) + Stavudine (C) | DispersibleTablet 30 mg(A)
+ 50 mg(B) + 6 mg(C) |
1 Tablet | 4.64 | 2400(E) | 28.07.2017 |
| 459 | L-Asparaginase | Powder for Injection 10000KU | Each Pack | 1,506.74 | 2058(E) | 30.06.2017 |
| 460 | L-Asparaginase | Powder for Injection 5000KU | Each Pack | 972.59 | 2058(E) | 30.06.2017 |
| 461 | Leflunomide | Tablet 10 mg | 1 Tablet | 9.40 | 2058(E) | 30.06.2017 |
| 462 | Leflunomide | Tablet 20 mg | 1 Tablet | 18.34 | 2058(E) | 30.06.2017 |
| 463 | Letrozole | Tablet 2.5 mg | 1 Tablet | 38.27 | 2059(E) | 30.06.2017 |
| 464 | Levetiracetam | Injection 100 mg/ml | 1 ml | 20.48 | 2058(E) | 30.06.2017 |
| 465 | Levetiracetam | ER Tablet 750mg | 1 Tablet | 16.32 | 2058(E) | 30.06.2017 |
| 466 | Levetiracetam | Tablet 250 mg | 1 Tablet | 5.49 | 2058(E) | 30.06.2017 |
| 467 | Levetiracetam | Tablet 500 mg | 1 Tablet | 11.10 | 2058(E) | 30.06.2017 |
| 468 | Levetiracetam | Tablet 750 mg | 1 Tablet | 17.06 | 2058(E) | 30.06.2017 |
| 469 | Levetiracetam | Oral Liquid 100 mg/ml | 1 ml | 3.42 | 2058(E) | 30.06.2017 |
| 470 | Levodopa (A) + Carbidopa (B) | CR Tablet 100mg(A) + 25
mg(B) |
1 Tablet | 3.13 | 2058(E) | 30.06.2017 |
| 471 | Levodopa (A) + Carbidopa (B) | Tablet 100 mg(A)+ 10 mg(B) | 1 Tablet | 1.46 | 2058(E) | 30.06.2017 |
| 472 | Levodopa (A) + Carbidopa (B) | Tablet 100 mg(A)+ 25 mg(B) | 1 Tablet | 2.12 | 2058(E) | 30.06.2017 |
| 473 | Levodopa (A) + Carbidopa (B) | Tablet 250 mg(A)+ 25 mg(B) | 1 Tablet | 3.51 | 2058(E) | 30.06.2017 |
| 474 | Levodopa (A) + Carbidopa (B) | CR Tablet 200mg(A) + 50
mg(B) |
1 Tablet | 3.86 | 2058(E) | 30.06.2017 |
| 475 | Levofloxacin | Tablet 250 mg | 1 Tablet | 4.14 | 2058(E) | 30.06.2017 |
| 476 | Levofloxacin | Tablet 750 mg | 1 Tablet | 10.26 | 2058(E) | 30.06.2017 |
| 477 | Levofloxacin | Tablet 500mg | 1 Tablet | 7.57 | 2401(E) | 28.07.2017 |
| 478 | Levonorgestrel | Tablet 0.75 mg | 1 Tablet | 21.61 | 2058(E) | 30.06.2017 |
| 479 | Levothyroxine | Tablet 100 mcg | 1 Tablet | 1.12 | 2058(E) | 30.06.2017 |
| 480 | Levothyroxine | Tablet 112mcg | 1 Tablet | 1.34 | 2397(E) | 28.07.2017 |
| 481 | Levothyroxine | Tablet 12.5 mcg | 1 Tablet | 1.27 | 2058(E) | 30.06.2017 |
| 482 | Levothyroxine | Tablet 125 mcg | 1 Tablet | 1.44 | 2058(E) | 30.06.2017 |
| 483 | Levothyroxine | Tablet 150 mcg | 1 Tablet | 1.45 | 2058(E) | 30.06.2017 |
| 484 | Levothyroxine | Tablet 25 mcg | 1 Tablet | 1.23 | 2058(E) | 30.06.2017 |
| 485 | Levothyroxine | Tablet 50 mcg | 1 Tablet | 0.92 | 2058(E) | 30.06.2017 |
| 486 | Levothyroxine | Tablet 62.5mcg | 1 Tablet | 1.31 | 2397(E) | 28.07.2017 |
| 487 | Levothyroxine | Tablet 75 mcg | 1 Tablet | 1.22 | 2058(E) | 30.06.2017 |
| 488 | Levothyroxine | Tablet 88 mcg | 1 Tablet | 1.44 | 2058(E) | 30.06.2017 |
| 489 | Lignocaine | Injection 2% | 1 ml | 0.92 | 2058(E) | 30.06.2017 |
| 490 | Lignocaine | Injection 2%(Preservative free for IV use) | 1 ml | 0.91 | 2058(E) | 30.06.2017 |
| 491 | Lignocaine | Injection 1% | Each Pack | 7.45 | 2058(E) | 30.06.2017 |
| 492 | Lignocaine | Topical Forms 2-5% | 1 gm or 1 ml | 1.01 | 650(E) | 13.02.2018 |
| 493 | Lignocaine | Injection 5%with 7.5% Glucose | 1 ml | 3.45 | 2058(E) | 30.06.2017 |
| 494 | Lignocaine (A) + Adrenaline (B) | Injection 1% (A)+ 1:200000 (5
mcg/ml) (B) |
1 ml | 0.42 | 3725(E) | 23.11.2017 |
| 495 | Lignocaine (A) + Adrenaline (B) | Injection 2% (A)+ 1:200000
(5mcg/ml) (B) |
1 ml | 0.86 | 2058(E) | 30.06.2017 |
| 496 | Linezolid | Tablet 600 mg | 1 Tablet | 31.01 | 2058(E) | 30.06.2017 |
| 497 | Lithium | Tablet 300 mg | 1 Tablet | 1.37 | 2058(E) | 30.06.2017 |
| 498 | Loperamide | Capsule 2 mg | 1 Capsule | 3.36 | 2058(E) | 30.06.2017 |
| 499 | Loperamide | Tablet 2 mg | 1 Tablet | 1.85 | 2058(E) | 30.06.2017 |
| 500 | Lopinavir (A) + Ritonavir (B) | Tablet 100 mg(A)+ 25 mg(B) | 1 Tablet | 22.07 | 2400(E) | 28.07.2017 |
| 501 | Lopinavir (A) + Ritonavir (B) | Tablet 200 mg(A)+ 50 mg(B) | 1 Tablet | 42.77 | 2400(E) | 28.07.2017 |
| 502 | Lorazepam | injection 2 mg/ml | 1 ml | 7.30 | 2058(E) | 30.06.2017 |
| 503 | Lorazepam | Tablet 1 mg | 1 Tablet | 1.94 | 2058(E) | 30.06.2017 |
| 504 | Lorazepam | Tablet 2 mg | 1 Tablet | 2.36 | 2058(E) | 30.06.2017 |
| 505 | Magnesium Sulphate | Injection 500 mg/ml | 1 ml | 4.48 | 2058(E) | 30.06.2017 |
| 506 | Mannitol | Injection 10% | 1 ml | 0.15129 | 3090(E) | 20.09.2017 |
| 507 | Mannitol | Injection 20% | 1 ml | 0.28 | 2058(E) | 30.06.2017 |
| 508 | Measles Rubbela Vaccine | Each Pack(0.5ml) | 82.98 | 3722(E) | 23.11.2017 | |
| 509 | Measles vaccine | Vaccine | Each Pack(0.5ml) | 47.86 | 3089(E) | 20.09.2017 |
| 510 | Mebendazole | Oral Liquid 100 mg/5ml | 1 ml | 0.73 | 2058(E) | 30.06.2017 |
| 511 | Mebendazole | Tablet 100 mg | 1 Tablet | 2.75 | 2058(E) | 30.06.2017 |
| 512 | Medroxyprogesteroneacetate | Tablet 10 mg | 1 Tablet | 5.05 | 2058(E) | 30.06.2017 |
| 513 | Mefenamic acid | Oral Liquid 100 mg/5ml | 1 ml | 0.49 | 2058(E) | 30.06.2017 |
| 514 | Mefenamic acid | Tablet 250 mg | 1 Tablet | 1.81 | 2058(E) | 30.06.2017 |
| 515 | Mefenamic acid | Tablet 500 mg | 1 Tablet | 2.30 | 2058(E) | 30.06.2017 |
| 516 | Mefloquine | Tablet 250 mg | 1 Tablet | 47.85 | 2058(E) | 30.06.2017 |
| 517 | Meglumine Diatrizoate | Injection 60%w/v | 1 ml | 7.76 | 2058(E) | 30.06.2017 |
| 518 | Meglumine Diatrizoate | Injection76%w/v | 1 ml | 8.96 | 2058(E) | 30.06.2017 |
| 519 | Melphalan | Tablet 2 mg | 1 Tablet | 92.08 | 2059(E) | 30.06.2017 |
| 520 | Melphalan | Tablet 5 mg | 1 Tablet | 157.93 | 2059(E) | 30.06.2017 |
| 521 | Mesna | Injection 100 mg/ml | 1 ml | 15.74 | 2059(E) | 30.06.2017 |
| 522 | Metformin | Immediate Release Tablet500 mg | 1 Tablet | 1.41 | 2058(E) | 30.06.2017 |
| 523 | Metformin | ControlledRelease Tablet
1000 mg |
1 Tablet | 3.42 | 2058(E) | 30.06.2017 |
| 524 | Metformin | ControlledRelease Tablet 500 mg | 1 Tablet | 1.79 | 2058(E) | 30.06.2017 |
| 525 | Metformin | controlled Released Tablet750 mg | 1 Tablet | 2.25 | 2058(E) | 30.06.2017 |
| 526 | Metformin | ImmediateRelease Tablet 1000 mg | 1 Tablet | 3.38 | 2058(E) | 30.06.2017 |
| 527 | Metformin | ImmediateRelease Tablet
750 mg |
1 Tablet | 2.85 | 2058(E) | 30.06.2017 |
| 528 | Methotrexate | Injection 25mg/ml | 1 ml | 45.14 | 2059(E) | 30.06.2017 |
| 529 | Methotrexate | Injection 50mg/ml | 1 ml | 37.60 | 2059(E) | 30.06.2017 |
| 530 | Methotrexate | Tablet 10 mg | 1 Tablet | 11.40 | 2059(E) | 30.06.2017 |
| 531 | Methotrexate | Tablet 2.5 mg | 1 Tablet | 4.49 | 2059(E) | 30.06.2017 |
| 532 | Methotrexate | Tablet 7.5 mg | 1 Tablet | 10.88 | 2059(E) | 30.06.2017 |
| 533 | Methotrexate | Tablet 5 mg | 1 Tablet | 7.86 | 2059(E) | 30.06.2017 |
| 534 | Methyldopa | Tablet 250 mg | 1 Tablet | 2.29 | 2058(E) | 30.06.2017 |
| 535 | Methyldopa | Tablet 500 mg | 1 Tablet | 4.39 | 2058(E) | 30.06.2017 |
| 536 | Methylergometrine | Injection 0.2 | 1 ml | 13.40 | 2058(E) | 30.06.2017 |
| mg/ml | ||||||
| 537 | Methylergometrine | Tablet 0.125 mg | 1 Tablet | 7.43 | 2058(E) | 30.06.2017 |
| 538 | Methylprednisolone | Tablet 16 mg | 1 Tablet | 8.37 | 2400(E) | 28.07.2017 |
| 539 | Methylprednisolone | Tablet 8 mg | 1 Tablet | 4.79 | 2400(E) | 28.07.2017 |
| 540 | Methylprednisolone | Injection40mg/ml | 1 ml | 50.65 | 650(E) | 13.02.2018 |
| 541 | Metoclopramide | Injection 5mg/ml (10 ml
Pack) |
1 ml | 1.33 | 2058(E) | 30.06.2017 |
| 542 | Metoclopramide | Injection 5mg/ml (2 ml Pack) | 1 ml | 2.20 | 2058(E) | 30.06.2017 |
| 543 | Metoclopramide | Oral Liquid 5mg/5ml | 1 ml | 0.41 | 2058(E) | 30.06.2017 |
| 544 | Metoclopramide | Tablet 10 mg | 1 Tablet | 1.09 | 2058(E) | 30.06.2017 |
| 545 | Metoprolol | Capsule 25 mg | 1 Capsule | 3.97 | 2058(E) | 30.06.2017 |
| 546 | Metoprolol | Capsule 50 mg | 1 Capsule | 6.05 | 2058(E) | 30.06.2017 |
| 547 | Metoprolol | SR Tablet 25 mg | 1 Tablet | 3.79 | 2058(E) | 30.06.2017 |
| 548 | Metoprolol | SR Tablet 50 mg | 1 Tablet | 5.42 | 2058(E) | 30.06.2017 |
| 549 | Metoprolol | Tablet 25 mg | 1 Tablet | 2.92 | 2058(E) | 30.06.2017 |
| 550 | Metoprolol | Tablet 50 mg | 1 Tablet | 4.42 | 2058(E) | 30.06.2017 |
| 551 | Metronidazole | Injection 500mg/100ml | 1 ml | 0.12557 | 352(E) | 23.01.2018 |
| 552 | Metronidazole | Oral Liquid 200 mg/5ml | 1 ml | 0.28 | 2058(E) | 30.06.2017 |
| 553 | Metronidazole | Tablet 200 mg | 1 Tablet | 0.40 | 2058(E) | 30.06.2017 |
| 554 | Metronidazole | Tablet 400 mg | 1 Tablet | 0.76 | 2058(E) | 30.06.2017 |
| 555 | Midazolam | Injection 1 mg/ml | 1 ml | 5.54 | 2058(E) | 30.06.2017 |
| 556 | Midazolam | Injection 5mg/ml | 1 ml | 13.62 | 2058(E) | 30.06.2017 |
| 557 | Midazolam | Tablet 7.5mg | 1 Tablet | 21.07 | 2601(E) | 11.08.2017 |
| 558 | Mifepristone | Tablet 200 mg | 1 Tablet | 301.96 | 2058(E) | 30.06.2017 |
| 559 | Misoprostol | Tablet 100 mcg | 1 Tablet | 8.17 | 2058(E) | 30.06.2017 |
| 560 | Misoprostol | Tablet 200 mcg | 1 Tablet | 15.67 | 2058(E) | 30.06.2017 |
| 561 | Morphine | Injection 10mg/ml | 1 ml | 22.29 | 2058(E) | 30.06.2017 |
| 562 | Morphine | Injection 15 mg/ml | 1 ml | 27.81 | 2058(E) | 30.06.2017 |
| 563 | Morphine | SR Tablet 30 mg | 1 Tablet | 5.11 | 3724(E) | 23.11.2017 |
| 564 | Morphine | Tablet 10 mg | 1 Tablet | 5.00 | 2058(E) | 30.06.2017 |
| 565 | Moxifloxacin | Tablet 400 mg | 1 Tablet | 23.78 | 2058(E) | 30.06.2017 |
| 566 | Mycophenolate mofetil | Tablet 250 mg | 1 Tablet | 38.97 | 2059(E) | 30.06.2017 |
| 567 | Mycophenolate mofetil | Tablet 500 mg | 1 Tablet | 77.09 | 2059(E) | 30.06.2017 |
| 568 | N-acetylcysteine | Injection 200mg/ml | 1 ml | 21.03 | 2058(E) | 30.06.2017 |
| 569 | N-acetylcysteine | Sachet 200mg | 1 gm | 8.44 | 269(E) | 16.01.2018 |
| 570 | Naloxone | injection 0.4 mg/ml | 1 ml | 81.43 | 2058(E) | 30.06.2017 |
| 571 | Natamycin | Drops 5% | 1 ml | 21.41 | 2058(E) | 30.06.2017 |
| 572 | Neostigmine | injection 0.5 | 1 ml | 4.09 | 2058(E) | 30.06.2017 |
| mg/ml | ||||||
| 573 | Neostigmine | Tablet 15 mg | 1 Tablet | 4.38 | 2058(E) | 30.06.2017 |
| 574 | Nevirapine | Oral Liquid 50 mg/5ml | 1 ml | 0.79 | 2400(E) | 28.07.2017 |
| 575 | Nevirapine | Tablet 200 mg | 1 Tablet | 13.44 | 2400(E) | 28.07.2017 |
| 576 | Nifedipine | Capsule 10 mg | 1 Capsule | 0.81 | 2058(E) | 30.06.2017 |
| 577 | Nifedipine | Tablet 10 mg | 1 Tablet | 1.16 | 2058(E) | 30.06.2017 |
| 578 | Nitrofurantoin | Oral Liquid 25 mg/5ml | 1 ml | 0.77 | 2058(E) | 30.06.2017 |
| 579 | Nitrofurantoin | Tablet 100 mg | 1 Tablet | 6.79 | 2058(E) | 30.06.2017 |
| 580 | Nitrofurantoin | Capsule 100mg | 1 Capsule | 7.38 | 2401(E) | 28.07.2017 |
| 581 | Noradrenaline | Injection 2 mg/ml | 1 ml | 24.18 | 2058(E) | 30.06.2017 |
| 582 | Norethisterone | Tablet 5 mg | 1 Tablet | 4.80 | 2058(E) | 30.06.2017 |
| 583 | Omeprazole | Capsule 10 mg | 1 Capsule | 1.99 | 2058(E) | 30.06.2017 |
| 584 | Omeprazole | Capsule 20 mg | 1 Capsule | 2.34 | 2058(E) | 30.06.2017 |
| 585 | Omeprazole | Capsule 40 mg | 1 Capsule | 4.63 | 2058(E) | 30.06.2017 |
| 586 | Omeprazole | Powder for oralLiquid 20 mg | 1 gm | 1.15 | 2058(E) | 30.06.2017 |
| 587 | Omeprazole | Tablet 10 mg | 1 Tablet | 4.16 | 2058(E) | 30.06.2017 |
| 588 | Omeprazole | Tablet 20 mg | 1 Tablet | 4.62 | 2058(E) | 30.06.2017 |
| 589 | Omeprazole | Tablet 40 mg | 1 Tablet | 7.83 | 2058(E) | 30.06.2017 |
| 590 | Ondansetron | Injection 2 mg/ml | 1 ml | 5.49 | 2058(E) | 30.06.2017 |
| 591 | Ondansetron | Oral Liquid 2mg/5ml | 1 ml | 1.06 | 2058(E) | 30.06.2017 |
| 592 | Ondansetron | Tablet 4 mg | 1 Tablet | 4.41 | 2058(E) | 30.06.2017 |
| 593 | Ondansetron | Tablet 8 mg | 1 Tablet | 9.16 | 2058(E) | 30.06.2017 |
| 594 | Oral Rehydration Salts | As Licensed | 1 gm | 0.74 | 2059(E) | 30.06.2017 |
| 595 | Oral Rehydration Salts | As Licensed | 1 ml | 0.13340 | 2059(E) | 30.06.2017 |
| 596 | Oxaliplatin | Injection100mg(as licensed) | Each Pack | 4,194.52 | 3722(E) | 23.11.2017 |
| 597 | Oxaliplatin | Injection 50mg(aslicensed) | Each Pack | 2,382.19 | 3723(E) | 23.11.2017 |
| 598 | Oxytocin | Injection 10 IU/ml | 1 ml | 36.97 | 2058(E) | 30.06.2017 |
| 599 | Oxytocin | Injection 5IU/ml | 1 ml | 16.56 | 2058(E) | 30.06.2017 |
| 600 | Paclitaxel | Injection 100 mg/16.7 ml | 1 ml | 209.47 | 2058(E) | 30.06.2017 |
| 601 | Paclitaxel | Injection 30 mg/5ml | 1 ml | 209.47 | 2058(E) | 30.06.2017 |
| 602 | Pantoprazole | Injection 40 mg | Each Pack | 41.79 | 2058(E) | 30.06.2017 |
| 603 | Para-aminosalicylic acid | Granules (Aslicensed) | 1 gm | 2.67 | 2601(E) | 11.08.2017 |
| 604 | Paracetamol | Oral Liquid 100mg/5ml | 1 ml | 0.48 | 2058(E) | 30.06.2017 |
| 605 | Paracetamol | Oral Liquid 120mg/5ml | 1 ml | 0.50 | 2058(E) | 30.06.2017 |
| 606 | Paracetamol | Oral Liquid 125 | 1 ml | 0.32 | 2058(E) | 30.06.2017 |
| mg/5ml | ||||||
| 607 | Paracetamol | Oral Liquid 150 mg/5ml | 1 ml | 0.55 | 2058(E) | 30.06.2017 |
| 608 | Paracetamol | Oral Liquid 250mg/5ml | 1 ml | 0.57 | 2058(E) | 30.06.2017 |
| 609 | Paracetamol | Oral Liquid 500 mg/5ml | 1 ml | 0.60 | 2058(E) | 30.06.2017 |
| 610 | Paracetamol | Oral Liquid 650 mg/5ml | 1 ml | 0.49 | 2058(E) | 30.06.2017 |
| 611 | Paracetamol | Tablet 500 mg | 1 Tablet | 0.85 | 2058(E) | 30.06.2017 |
| 612 | Paracetamol | Tablet 650 mg | 1 Tablet | 1.73 | 2058(E) | 30.06.2017 |
| 613 | Paracetamol | Injection 150 mg/ml | Each Pack (0.5 ml) | 2.88 | 2058(E) | 30.06.2017 |
| 614 | Paracetamol | Injection 150mg/ml | Each Pack (1 ml) | 3.94 | 2058(E) | 30.06.2017 |
| 615 | Paracetamol | Injection 150 mg/ml | Each Pack (2 ml) | 6.05 | 2058(E) | 30.06.2017 |
| 616 | Paracetamol | Injection 150mg/ml | Each Pack (3 ml) | 8.17 | 2058(E) | 30.06.2017 |
| 617 | Paracetamol | Injection 150 mg/ml | Each Pack (4 ml) | 10.29 | 2058(E) | 30.06.2017 |
| 618 | Paracetamol | Injection 150mg/ml | Each Pack (5ml) | 12.41 | 2058(E) | 30.06.2017 |
| 619 | Paracetamol | Injection 150mg/ml | Each Pack (7ml) | 16.64 | 2058(E) | 30.06.2017 |
| 620 | Paracetamol | Suppository 170mg | EachSuppository | 7.22 | 2058(E) | 30.06.2017 |
| 621 | Paracetamol | Suppository 80mg | EachSuppository | 6.11 | 2058(E) | 30.06.2017 |
| 622 | Pegylated interferon alfa 2a | Injection 180mcg | Each Pack | 7,426.96 | 2058(E) | 30.06.2017 |
| 623 | Pegylated interferon alfa 2b | Injection 100mcg | Each Pack | 14,684.00 | 2058(E) | 30.06.2017 |
| 624 | Pegylated interferon alfa 2b | Injection 120mcg | Each Pack | 13,674.96 | 2058(E) | 30.06.2017 |
| 625 | Pegylated interferon alfa 2b | Injection 80mcg | Each Pack | 11,739.44 | 2058(E) | 30.06.2017 |
| 626 | Penicillamine | Capsule 250 mg | 1 Capsule | 13.95 | 2059(E) | 30.06.2017 |
| 627 | Permethrin | Cream 1% | 1 gm | 1.51 | 2058(E) | 30.06.2017 |
| 628 | Permethrin | Cream 5% | 1 gm | 1.63 | 2058(E) | 30.06.2017 |
| 629 | Permethrin | Gel 5% | 1 gm | 1.37 | 2058(E) | 30.06.2017 |
| 630 | Permethrin | Lotion 1% | 1 gm | 0.85 | 2058(E) | 30.06.2017 |
| 631 | Permethrin | Lotion 5% | 1 ml | 0.91 | 2058(E) | 30.06.2017 |
| 632 | Pheniramine | Injection 22.75 mg/ml (2ml) | 1 ml | 1.43 | 2058(E) | 30.06.2017 |
| 633 | Pheniramine | Injection 22.75mg/ml(10ml) | 1 ml | 1.06 | 2058(E) | 30.06.2017 |
| 634 | Phenobarbitone | Injection 200mg/ml | 1 ml | 17.99 | 2058(E) | 30.06.2017 |
| 635 | Phenobarbitone | Tablet 30 mg | 1 Tablet | 1.15 | 2058(E) | 30.06.2017 |
| 636 | Phenobarbitone | Tablet 60 mg | 1 Tablet | 1.66 | 2058(E) | 30.06.2017 |
| 637 | Phenobarbitone | Oral liquid 20mg/5 ml | 1 ml | 0.41 | 884(E) | 28.02.2018 |
| 638 | Phenylephrine | Drop 10% | 1 ml | 7.59 | 2397(E) | 28.07.2017 |
| 639 | Phenylephrine | Drops 5% | 1 ml | 3.50 | 3914(E) | 18.12.2017 |
| 640 | Phenytoin | Capsule 100 mg | 1 Capsule | 1.43 | 2058(E) | 30.06.2017 |
| 641 | Phenytoin | Capsule 300 mg | 1 Capsule | 3.58 | 2058(E) | 30.06.2017 |
| 642 | Phenytoin | ERCapsule 300mg | 1 Capsule | 3.58 | 2058(E) | 30.06.2017 |
| 643 | Phenytoin | injection 25mg/ml | 1 ml | 2.36 | 2058(E) | 30.06.2017 |
| 644 | Phenytoin | Injection 50mg/ml | 1 ml | 5.04 | 2058(E) | 30.06.2017 |
| 645 | Phenytoin | Oral Liquid 125mg/5ml | 1 ml | 0.84 | 2058(E) | 30.06.2017 |
| 646 | Phenytoin | Oral Liquid 30mg/5ml | 1 ml | 0.28 | 2058(E) | 30.06.2017 |
| 647 | Phenytoin | ER Tablet 300 mg | 1 Tablet | 5.45 | 2058(E) | 30.06.2017 |
| 648 | Phenytoin | Tablet 100 mg | 1 Tablet | 1.46 | 2058(E) | 30.06.2017 |
| 649 | Phenytoin | Tablet 300 mg | 1 Tablet | 4.45 | 2058(E) | 30.06.2017 |
| 650 | Phenytoin | Tablet 50 mg | 1 Tablet | 0.70 | 2058(E) | 30.06.2017 |
| 651 | Phytomenadione (Vitamin K1) | Injection10mg/ml | 1 ml | 44.94 | 3723(E) | 23.11.2017 |
| 652 | Pilocarpine | Drops 2% | 1 ml | 9.49 | 2058(E) | 30.06.2017 |
| 653 | Pilocarpine | Drops 4% | 1 ml | 10.96 | 3725(E) | 23.11.2017 |
| 654 | Piperacillin (A) + Tazobactam(B) | Powder forInjection 1 g (A)
+ 125 mg(B) |
Each Pack | 82.97 | 2058(E) | 30.06.2017 |
| 655 | Piperacillin (A) + Tazobactam (B) | Powder for Injection 2 g (A)+ 250 mg(B) | Each Pack | 196.28 | 2058(E) | 30.06.2017 |
| 656 | Piperacillin (A) + Tazobactam (B) | Powder for Injection 4 g (A)+ 500 mg(B) | Each Pack | 412.31 | 2058(E) | 30.06.2017 |
| 657 | Potassium chloride | Injection 150mg/ml | 1 ml | 2.28 | 2058(E) | 30.06.2017 |
| 658 | Potassium chloride | Oral Liquid 500mg/5ml | 1 ml | 0.27 | 2058(E) | 30.06.2017 |
| 659 | Potassium permanganate | Crystals fortopical solution | 1 gm | 0.41 | 3725(E) | 23.11.2017 |
| 660 | Povidone iodine | Solution 4% | 1 ml | 1.63 | 2058(E) | 30.06.2017 |
| 661 | Povidone iodine | Solution 7.5% | 1 ml | 0.73 | 2058(E) | 30.06.2017 |
| 662 | Povidone Iodine | Solution 10% | 1 ml | 0.79 | 650(E) | 13.02.2018 |
| 663 | Povidone Iodine | Solution 5% | 1 ml | 0.36 | 2401(E) | 28.07.2017 |
| 664 | Pralidoxime chloride (2-PAM) | Injection 25 mg/ml | 1 ml | 3.50 | 2058(E) | 30.06.2017 |
| 665 | Prednisolone | Drops 1% | 1 ml | 3.15 | 2058(E) | 30.06.2017 |
| 666 | Prednisolone | Injection 20mg/2ml | 1 ml | 3.36 | 2058(E) | 30.06.2017 |
| 667 | Prednisolone | Oral Liquid 15mg/ 5ml | 1 ml | 0.71 | 2058(E) | 30.06.2017 |
| 668 | Prednisolone | Oral Liquid 5mg/ 5ml | 1 ml | 0.40 | 2058(E) | 30.06.2017 |
| 669 | Prednisolone | Tablet 10 mg | 1 Tablet | 0.91 | 2058(E) | 30.06.2017 |
| 670 | Prednisolone | Tablet 20 mg | 1 Tablet | 1.83 | 2058(E) | 30.06.2017 |
| 671 | Prednisolone | Tablet 40 mg | 1 Tablet | 2.64 | 2058(E) | 30.06.2017 |
| 672 | Prednisolone | Tablet 5 mg | 1 Tablet | 0.53 | 2058(E) | 30.06.2017 |
| 673 | Premix Insulin 30:70 Injection (Regular:NPH) | Injection 40 IU/ml | 1 ml | 14.13 | 2059(E) | 30.06.2017 |
| 674 | Prilocaine (A) + Lignocaine(B) | Cream 2.5% (A)+ 2.5% (B ) | 1 gm | 19.19 | 2058(E) | 30.06.2017 |
| 675 | Primaquine | Tablet 15 mg | 1 Tablet | 4.30 | 2058(E) | 30.06.2017 |
| 676 | Primaquine | Tablet 2.5 mg | 1 Tablet | 1.46 | 2058(E) | 30.06.2017 |
| 677 | Primaquine | Tablet 7.5 mg | 1 Tablet | 1.99 | 2058(E) | 30.06.2017 |
| 678 | Procarbazine | Capsule 50 mg | 1 Capsule | 32.45 | 2059(E) | 30.06.2017 |
| 679 | Procarbazine | Tablet 50 mg | 1 Tablet | 44.21 | 2059(E) | 30.06.2017 |
| 680 | Proparacaine | Drops 0.5% | 1 ml | 9.36 | 2058(E) | 30.06.2017 |
| 681 | Propofol | Injection 10mg/ml | 1 ml | 6.78 | 2058(E) | 30.06.2017 |
| 682 | Propranolol | Capsule 40 mg | 1 Capsule | 3.57 | 2058(E) | 30.06.2017 |
| 683 | Propranolol | Capsule 80 mg | 1 Capsule | 5.42 | 2058(E) | 30.06.2017 |
| 684 | Propranolol | Tablet 10 mg | 1 Tablet | 1.09 | 2058(E) | 30.06.2017 |
| 685 | Propranolol | Tablet 40 mg | 1 Tablet | 2.59 | 2058(E) | 30.06.2017 |
| 686 | Propranolol | Tablet 80 mg | 1 Tablet | 4.69 | 2058(E) | 30.06.2017 |
| 687 | Protamine | Injection 10 mg/ml | 1 ml | 8.68 | 2059(E) | 30.06.2017 |
| 688 | Pyrazinamide | Oral Liquid 250mg/5ml | 1 ml | 0.58 | 2059(E) | 30.06.2017 |
| 689 | Pyrazinamide | Tablet 1000 mg | 1 Tablet | 8.85 | 2059(E) | 30.06.2017 |
| 690 | Pyrazinamide | Tablet 1500 mg | 1 Tablet | 9.80 | 2059(E) | 30.06.2017 |
| 691 | Pyrazinamide | Tablet 500 mg | 1 Tablet | 3.97 | 2059(E) | 30.06.2017 |
| 692 | Pyrazinamide | Tablet 750 mg | 1 Tablet | 6.04 | 2059(E) | 30.06.2017 |
| 693 | Pyridoxine | Tablet 100 mg | 1 Tablet | 5.25 | 2058(E) | 30.06.2017 |
| 694 | Pyridoxine | Tablet 10mg | 1 Tablet | 0.10852 | 3090(E) | 20.09.2017 |
| 695 | Quinine | Injection 300 mg/ml | 1 ml | 9.54 | 2059(E) | 30.06.2017 |
| 696 | Quinine | Tablet 300 mg | 1 Tablet | 5.37 | 2059(E) | 30.06.2017 |
| 697 | Rabies Vaccine | Each Pack | 320.74 | 2059(E) | 30.06.2017 | |
| 698 | Raltegravir | Tablet 400 mg | 1 Tablet | 146.88 | 2059(E) | 30.06.2017 |
| 699 | Ramipril | Capsule 2.5 mg | 1 Capsule | 4.44 | 2058(E) | 30.06.2017 |
| 700 | Ramipril | Capsule 5 mg | 1 Capsule | 6.93 | 2058(E) | 30.06.2017 |
| 701 | Ramipril | Tablet 2.5 mg | 1 Tablet | 4.67 | 2058(E) | 30.06.2017 |
| 702 | Ramipril | Tablet 5 mg | 1 Tablet | 7.36 | 2058(E) | 30.06.2017 |
| 703 | Ranitidine | Oral Liquid 75 mg/5ml | 1 ml | 0.55 | 2058(E) | 30.06.2017 |
| 704 | Ranitidine | Tablet 150 mg | 1 Tablet | 0.69 | 2058(E) | 30.06.2017 |
| 705 | Ranitidine | Injection25mg/ml | 1 ml | 1.52 | 2401(E) | 28.07.2017 |
| 706 | Ribavirin | Capsule 200 mg | 1 Capsule | 78.51 | 2059(E) | 30.06.2017 |
| 707 | Rifabutin | Capsule 150mg | 1 Capsule | 37.23 | 3088(E) | 11.08.2017 |
| 708 | Rifabutin | Tablet 150mg | 1 Tablet | 34.77 | 2601(E) | 11.08.2017 |
| 709 | Rifampicin | Capsule 150 mg | 1 Capsule | 1.88 | 2058(E) | 30.06.2017 |
| 710 | Rifampicin | Capsule 300 mg | 1 Capsule | 3.57 | 2058(E) | 30.06.2017 |
| 711 | Rifampicin | Capsule 450 mg | 1 Capsule | 4.63 | 2058(E) | 30.06.2017 |
| 712 | Rifampicin | Capsule 600 mg | 1 Capsule | 10.89 | 2058(E) | 30.06.2017 |
| 713 | Rifampicin | Oral Liquid 100mg/5ml | 1 ml | 0.36 | 2058(E) | 30.06.2017 |
| 714 | Rifampicin | Tablet 150 mg | 1 Tablet | 1.52 | 2058(E) | 30.06.2017 |
| 715 | Rifampicin | Tablet 300 mg | 1 Tablet | 2.75 | 2058(E) | 30.06.2017 |
| 716 | Ringer Lactate | Injection 1000ml | Each Pack | 74.87 | 2401(E) | 28.07.2017 |
| 717 | Ringer Lactate | Injection 100ml | Each Pack | 19.60 | 2401(E) | 28.07.2017 |
| 718 | Ringer Lactate | Injection 250ml | Each Pack | 33.42 | 2401(E) | 28.07.2017 |
| 719 | Ringer Lactate | Injection 500ml | Each Pack | 42.59 | 2401(E) | 28.07.2017 |
| 720 | Risperidone | Oral Liquid 1 mg/ml | 1 ml | 1.81 | 2058(E) | 30.06.2017 |
| 721 | Risperidone | Tablet 1 mg | 1 Tablet | 2.89 | 2058(E) | 30.06.2017 |
| 722 | Risperidone | Tablet 2 mg | 1 Tablet | 4.62 | 2058(E) | 30.06.2017 |
| 723 | Risperidone | Tablet 4 mg | 1 Tablet | 9.39 | 2058(E) | 30.06.2017 |
| 724 | Ritonavir | Capsule 100 mg | 1 Capsule | 26.49 | 2058(E) | 30.06.2017 |
| 725 | Ritonavir | Tablet 100 mg | 1 Tablet | 27.24 | 2058(E) | 30.06.2017 |
| 726 | Rituximab | Injection 10mg/ml | 1 ml | 711.90 | 2400(E) | 28.07.2017 |
| 727 | Salbutamol | Capsule 4 mg | 1 Capsule | 0.57 | 2058(E) | 30.06.2017 |
| 728 | Salbutamol | Inhalation (MDI/DPI) 100mcg/dose | 1 Dose | 0.37 | 2058(E) | 30.06.2017 |
| 729 | Salbutamol | Oral Liquid 2mg/5ml | 1 ml | 0.14481 | 2058(E) | 30.06.2017 |
| 730 | Salbutamol | Respiratorsolution for use in Nebulizer 5
mg/ml |
1 ml | 0.66 | 2058(E) | 30.06.2017 |
| 731 | Salbutamol | Tablet 2 mg | 1 Tablet | 0.15 | 2058(E) | 30.06.2017 |
| 732 | Salbutamol | Tablet 4 mg | 1 Tablet | 0.17197 | 2058(E) | 30.06.2017 |
| 733 | Salicylic acid | Ointment 6% | 1 gm | 1.69 | 2058(E) | 30.06.2017 |
| 734 | Sevoflurane | Inhalation | 1 ml | 24.95 | 3723(E) | 23.11.2017 |
| 735 | Silver Sulphadiazine | Cream 1% | 1 gm | 0.28 | 3089(E) | 20.09.2017 |
| 736 | Snake venom antiserum- Lyophilized polyvalent | Powder for Injection | 10 ml Pack | 523.00 | 2058(E) | 30.06.2017 |
| 737 | Snake Venom Antiserum- Soluble/ Liquid Polyvalent | Injection | Each Pack (10 ML) | 406.14 | 884(E) | 28.02.2018 |
| 738 | Sodium Bicarbonate | Injection 7.5% | 1 ml | 1.31 | 2058(E) | 30.06.2017 |
| 739 | Sodium Bicarbonate | Injection 8.4% | 1 ml | 1.21 | 2058(E) | 30.06.2017 |
| 740 | Sodium Chloride | Injection 0.9% | 1000ml Glass | 55.70 | 2058(E) | 30.06.2017 |
| 741 | Sodium Chloride | Injection 0.9% | 1000ml Non-Glass | 43.94 | 2058(E) | 30.06.2017 |
| 742 | Sodium Chloride | Injection 0.9% | 100ml Glass | 16.48 | 2058(E) | 30.06.2017 |
| 743 | Sodium Chloride | Injection 0.9% | 100ml Non-Glass | 14.84 | 2058(E) | 30.06.2017 |
| 744 | Sodium Chloride | Injection 0.9% | 250ml Glass | 22.21 | 2058(E) | 30.06.2017 |
| 745 | Sodium Chloride | Injection 0.9% | 250ml Non-Glass | 19.95 | 2058(E) | 30.06.2017 |
| 746 | Sodium Chloride | Injection 0.9% | 500ml Glass | 30.88 | 2058(E) | 30.06.2017 |
| 747 | Sodium Chloride | Injection 0.9% | 500ml Non-Glass | 26.29 | 2058(E) | 30.06.2017 |
| 748 | Sodium Nitroprusside | Injection 10 mg/ml | 1 ml | 24.23 | 2058(E) | 30.06.2017 |
| 749 | Sodium Valproate | Injection 100 mg/ml | 1 ml | 5.41 | 2058(E) | 30.06.2017 |
| 750 | Sodium Valproate | Oral Liquid 200 | 1 ml | 0.55 | 2058(E) | 30.06.2017 |
| mg/5ml | ||||||
| 751 | Sodium Valproate | CR Tablets 300mg | 1 Tablet | 5.91 | 650(E) | 13.02.2018 |
| 752 | Sodium Valproate | CR Tablets500mg | 1 Tablet | 9.15 | 650(E) | 13.02.2018 |
| 753 | Sodium Valproate | Tablet 200 mg | 1 Tablet | 2.91 | 2058(E) | 30.06.2017 |
| 754 | Sodium Valproate | Tablet 300 mg | 1 Tablet | 3.73 | 2058(E) | 30.06.2017 |
| 755 | Sodium Valproate | Tablet 500 mg | 1 Tablet | 6.51 | 2058(E) | 30.06.2017 |
| 756 | Sofosbuvir | Tablet 400 mg | 1 Tablet | 626.48 | 2058(E) | 30.06.2017 |
| 757 | Somatostatin | Powder for Injection 3 mg | Each pack | 1,487.48 | 2059(E) | 30.06.2017 |
| 758 | Spironolactone | Tablet 25 mg | 1 Tablet | 1.79 | 2058(E) | 30.06.2017 |
| 759 | Spironolactone | Tablet 50 mg | 1 Tablet | 3.57 | 2058(E) | 30.06.2017 |
| 760 | Stavudine (A) + Lamivudine (B) | Tablet 30 mg(A)+150 mg(B) | 1 Tablet | 8.96 | 2400(E) | 28.07.2017 |
| 761 | Streptokinase | Injection15,00,000 IU | Each Pack | 1,680.08 | 2058(E) | 30.06.2017 |
| 762 | Streptokinase | Injection7,50,000 IU | Each Pack | 1,181.65 | 2058(E) | 30.06.2017 |
| 763 | Streptomycin | Powder forInjection 750 mg | Each Pack | 8.24 | 2059(E) | 30.06.2017 |
| 764 | Streptomycin | Powder forInjection 1 gm | Each Pack | 9.79 | 2059(E) | 30.06.2017 |
| 765 | Succinyl Choline | Injection 50mg/ml | 1 ml | 4.54 | 2058(E) | 30.06.2017 |
| 766 | Sucralfate | Oral Liquid 1 g | 1 ml | 0.66 | 2058(E) | 30.06.2017 |
| 767 | Sulfasalazine | Tablet 500 mg | 1 Tablet | 3.74 | 2058(E) | 30.06.2017 |
| 768 | Sumatriptan | Tablet 25mg | 1 Tablet | 31.25 | 3088(E) | 11.08.2017 |
| 769 | Sumatriptan | Tablet 50mg | 1 Tablet | 48.37 | 3088(E) | 11.08.2017 |
| 770 | Surfactant | Suspension for intratrachealinstillation (As liensed) | Per mg of Phospholipids in the pack | 62.78 | 3722(E) | 23.11.2017 |
| 771 | Tacrolimus | Capsule 0.5 mg | 1 Capsule | 20.75 | 2058(E) | 30.06.2017 |
| 772 | Tacrolimus | Capsule 1 mg | 1 Capsule | 36.67 | 2058(E) | 30.06.2017 |
| 773 | Tacrolimus | Capsule 2 mg | 1 Capsule | 71.83 | 2058(E) | 30.06.2017 |
| 774 | Tamoxifen | Tablet 10 mg | 1 Tablet | 2.42 | 2058(E) | 30.06.2017 |
| 775 | Tamoxifen | Tablet 20 mg | 1 Tablet | 2.73 | 2058(E) | 30.06.2017 |
| 776 | Telmisartan | Tablet 20 mg | 1 Tablet | 3.42 | 2058(E) | 30.06.2017 |
| 777 | Telmisartan | Tablet 40 mg | 1 Tablet | 6.19 | 2058(E) | 30.06.2017 |
| 778 | Telmisartan | Tablet 80 mg | 1 Tablet | 9.43 | 2058(E) | 30.06.2017 |
| 779 | Temozolomide | Capsule 100 mg | 1 Capsule | 1,955.67 | 2058(E) | 30.06.2017 |
| 780 | Temozolomide | Capsule 20 mg | 1 Capsule | 559.80 | 2058(E) | 30.06.2017 |
| 781 | Temozolomide | Capsule 250 mg | 1 Capsule | 4,398.65 | 2058(E) | 30.06.2017 |
| 782 | Temozolomide | Tablet 100 mg | 1 Tablet | 1,451.66 | 2058(E) | 30.06.2017 |
| 783 | Temozolomide | Tablet 20 mg | 1 Tablet | 351.71 | 2058(E) | 30.06.2017 |
| 784 | Temozolomide | Tablet 250 mg | 1 Tablet | 3,593.01 | 2058(E) | 30.06.2017 |
| 785 | Tenofovir | Tablet 300 mg | 1 Tablet | 44.29 | 2058(E) | 30.06.2017 |
| 786 | Tenofovir (A) + Lamivudine (B) | Tablet 300 mg(A)+ 300 mg(B) | 1 Tablet | 42.13 | 2059(E) | 30.06.2017 |
| 787 | Tenofovir (A) + Lamivudine | Tablet 300 mg(A) | 1 Tablet | 96.74 | 2059(E) | 30.06.2017 |
| (B) + Efavirenz (C) | + 300 mg(B) + 600 mg(C) | |||||
| 788 | Tetanus Toxoid | Injection | Each Pack(0.5ml) | 5.83 | 2059(E) | 30.06.2017 |
| 789 | Tetanus Toxoid | Injection | Each Pack (5ml) | 25.75 | 2059(E) | 30.06.2017 |
| 790 | Thalidomide | Capsule 100 mg | 1 Capsule | 57.34 | 2058(E) | 30.06.2017 |
| 791 | Thalidomide | Capsule 50 mg | 1 Capsule | 33.13 | 2058(E) | 30.06.2017 |
| 792 | Thiamine | Injection 100 mg/ml | 1 ml | 23.28 | 2058(E) | 30.06.2017 |
| 793 | Thiamine | Tablet 100 mg | 1 Tablet | 3.52 | 2058(E) | 30.06.2017 |
| 794 | Thiopentone | Powder forInjection 0.5 gm | Each Pack | 38.23 | 2058(E) | 30.06.2017 |
| 795 | Thiopentone | Powder forInjection 1 gm | Each Pack | 46.39 | 2058(E) | 30.06.2017 |
| 796 | Timolol | Drops 0.25% | 1 ml | 4.84 | 2058(E) | 30.06.2017 |
| 797 | Timolol | Drops 0.5% | 1 ml | 11.19 | 2058(E) | 30.06.2017 |
| 798 | Tiotropium | Inhalation (DPI) 18 mcg/dose | 1 Dose | 2.32 | 2058(E) | 30.06.2017 |
| 799 | Tiotropium | Inhalation (MDI)9 mcg/dose | 1 Dose | 2.18 | 2058(E) | 30.06.2017 |
| 800 | Tramadol | Capsule 50 mg | 1 Capsule | 4.24 | 2058(E) | 30.06.2017 |
| 801 | Tramadol | Injection 50mg/ml (20 ml
Pack) |
1 ml | 1.17 | 2058(E) | 30.06.2017 |
| 802 | Tramadol | Injection 50mg/ml (upto 2 ml Pack) | 1 ml | 9.83 | 2058(E) | 30.06.2017 |
| 803 | Tramadol | Tablet 100 mg | 1 Tablet | 18.46 | 2058(E) | 30.06.2017 |
| 804 | Tramadol | Tablet 50 mg | 1 Tablet | 7.57 | 2058(E) | 30.06.2017 |
| 805 | Tramadol | Capsule 100 mg | 1 Capsule | 9.01 | 2058(E) | 30.06.2017 |
| 806 | Tranexamic acid | Injection 100mg/ml | 1 ml | 14.02 | 2059(E) | 30.06.2017 |
| 807 | Tranexamic acid | Tablet 500 mg | 1 Tablet | 16.30 | 2059(E) | 30.06.2017 |
| 808 | Trastuzumab | Injection 440 mg/50 ml | Each Pack | 56,458.85 | 2058(E) | 30.06.2017 |
| 809 | Trihexyphenidyl | Tablet 2 mg | 1 Tablet | 1.14 | 2058(E) | 30.06.2017 |
| 810 | Tropicamide | Eye Drop 1% | 1 ml | 9.44 | 2058(E) | 30.06.2017 |
| 811 | Vancomycin | Powder forInjection 1 gm | Each Pack | 446.68 | 2059(E) | 30.06.2017 |
| 812 | Vancomycin | Powder forInjection 250 mg | Each Pack | 208.12 | 2059(E) | 30.06.2017 |
| 813 | Vancomycin | Powder forInjection 500 mg | Each Pack | 268.67 | 2059(E) | 30.06.2017 |
| 814 | Vecuronium | Powder forInjection 10 mg | Each Pack | 177.46 | 2059(E) | 30.06.2017 |
| 815 | Vecuronium | Powder for Injection 4 mg | Each Pack | 85.65 | 2059(E) | 30.06.2017 |
| 816 | Verapamil | Injection 2.5mg/ml | 1 ml | 1.09 | 2058(E) | 30.06.2017 |
| 817 | Verapamil | Tablet 40 mg | 1 Tablet | 0.70 | 2058(E) | 30.06.2017 |
| 818 | Verapamil | Tablet 80 mg | 1 Tablet | 1.30 | 2058(E) | 30.06.2017 |
| 819 | Vinblastine | Injection 1mg/ml | 1 ml | 19.26 | 3089(E) | 20.09.2017 |
| 820 | Vincristine | Injection 1mg/ml | 1 ml | 48.91 | 2059(E) | 30.06.2017 |
| 821 | Vitamin A | Capsule 50000 IU | 1 Capsule | 0.52 | 3090(E) | 20.09.2017 |
| 822 | Vitamin A | Injection 50000 IU/ml | 1 ml | 1.79 | 2058(E) | 30.06.2017 |
| 823 | Vitamin A | Oral liquid100000 IU/ml | 1 ml | 0.49 | 3091(E) | 20.09.2017 |
| 824 | Vitamin A | Tablet 50000 IU | 1 Tablet | 0.66 | 2058(E) | 30.06.2017 |
| 825 | Warfarin | Tablet 1 mg | 1 Tablet | 2.27 | 2058(E) | 30.06.2017 |
| 826 | Warfarin | Tablet 2 mg | 1 Tablet | 2.50 | 2058(E) | 30.06.2017 |
| 827 | Warfarin | Tablet 3 mg | 1 Tablet | 2.93 | 2058(E) | 30.06.2017 |
| 828 | Warfarin | Tablet 5 mg | 1 Tablet | 2.22 | 2058(E) | 30.06.2017 |
| 829 | Water for Injection | Injection | Each Pack (10ml) | 2.19 | 2058(E) | 30.06.2017 |
| 830 | Water for Injection | Injection | Each Pack (5ml) | 2.11 | 2058(E) | 30.06.2017 |
| 831 | White Petrolatum | Jelly 100% | 1 gm | 0.08720 | 3091(E) | 20.09.2017 |
| 832 | Xylometazoline | Nasal Drops0.05% | 1 ml | 3.69 | 2058(E) | 30.06.2017 |
| 833 | Xylometazoline | Nasal Drops0.1% | 1 ml | 4.88 | 2058(E) | 30.06.2017 |
| 834 | Zidovudine | Capsule 300 mg | 1 Capsule | 1.94 | 2059(E) | 30.06.2017 |
| 835 | Zidovudine | Tablet 300 mg | 1 Tablet | 13.38 | 2059(E) | 30.06.2017 |
| 836 | Zidovudine (A) + Lamivudine(B) + Nevirapine (C) | Tablet 300 mg(A)+ 150 mg(B) + 200 mg(C) | 1 Tablet | 18.91 | 2400(E) | 28.07.2017 |
| 837 | Zinc sulphate | DispersibleTablet 20mg | 1 Tablet | 2.94 | 2397(E) | 28.07.2017 |
| 838 | Zoledronic Acid | Powder for Injection 4 mg | Each Pack | 3,943.16 | 2059(E) | 30.06.2017 |
| 839 | Zolpidem | Capsule 5 mg | 1 Capsule | 6.90 | 2058(E) | 30.06.2017 |
| 840 | Zolpidem | Tablet 10 mg | 1 Tablet | 7.92 | 2058(E) | 30.06.2017 |
| 841 | Zolpidem | Tablet 5 mg | 1 Tablet | 5.05 | 2058(E) | 30.06.2017 |
Notes:-
- The ceiling prices are applicable with effect from 4.2018 (ceiling prices are inclusive of Wholesale Price Index (WPI) @3.43812% for the year 2017 over 2016).
- In respect of Gentamycin injection 40mg/ml, Dexamethasone injection 4mg/ml, Paracetamol injection 150mg/ml, Glucose Injection 5%, Sodium chloride Injection 9%, Glucose + Sodium chloride Injection 5%+0.9%, Water for Injection, Dicyclomine Injection 10mg/ml, Snake venom antiserum, Pheniramine Injection 22.75 mg/ml and Tetanus Toxoid injection, for any other pack size manufactured, the manufacturer shall approach NPPA for specific price approval for its formulations along with the relevant market data duly authenticated for fixation of the ceiling price. The formulation of Sodium Valproate includes combination of Sodium Valproate and Valproic Acid both together corresponding to Sodium Valproate of the stated strength.
- In respect of any other scheduled formulation, for which ceiling price is not mentioned above, the manufacturer shall approach the NPPA for specific price approval for its formulations along with the relevant market data duly authenticated for fixation of the ceiling
- All manufacturers of scheduled formulations, selling branded or generic or both the versions of scheduled formulations at price higher than the ceiling price (plus goods and services taxes as applicable) so fixed and notified by the Government, shall revise the prices of all such formulations downward not exceeding the ceiling price specified in column (5) in the above table plus goods and services taxes as applicable, if
- All the existing manufacturers of above mentioned scheduled formulations having MRP lower than the ceiling price specified in column (5) in the above table (plus goods and services taxes as applicable, if any), may revise the existing MRP of their formulations, on the basis of WPI @ 43812% for year 2017 in accordance with paragraph 16(2) of DPCO, 2013, read with para 13(2) of DPCO, 2013.
- The manufacturers may add goods and services taxes only if they have paid actually or if it is payable to the Government on the ceiling price mentioned in column (5) of the above said
- The ceiling price for a pack of the scheduled formulation shall be arrived at by the concerned manufacturer in accordance with the ceiling price specified in column (5) of the above table as per provisions contained in paragraph 11 of the Drugs (Prices Control) Order, The manufacturer shall issue a price list in Form–V from date of Notification as per paragraph 24 of the DPCO, 2013 to NPPA through IPDMS and submit a copy to State Drug Controller and dealers.
- As per para 24(4) of DPCO 2013, every retailer and dealer shall display price list and the supplementary price list, if any, as furnished by the manufacturer, on a conspicuous part of the premises where he carries on business in a manner so as to be easily accessible to any person wishing to consult the
- Where an existing manufacturer of scheduled formulation with dosage or strength or both as specified in the above table launches a new drug as per paragraph 2 (u) of the DPCO, 2013 such existing manufacturer shall apply for prior price approval of such new drug to the NPPA in Form I as specified under Schedule-II of the DPCO,
- The manufacturers of above said scheduled formulations shall furnish quarterly return to the NPPA, in respect of production/import and sale of scheduled formulations in Form -III of Schedule-II of the DPCO, 2013 through Any manufacturer intending to discontinue production of above said scheduled formulation shall furnish information to the NPPA, in respect of discontinuation of production and/or import of scheduled formulation in Form -IV of Schedule-II of the DPCO, 2013 at least six months prior to the intended date of discontinuation.
- The manufacturers not complying with the ceiling price and notes specified hereinabove shall be liable to deposit the overcharged amount along with interest thereon under the provisions of the Drugs (Prices Control) Order, 2013 read with Essential Commodities Act,
- The notification under para 19 of DPCO 2013 vide SO 3090(E) dated 09.2017, 3091(E) dated 20.09.2017, SO 3723(E) dated 23.11.2017, SO 3724(E) dated 23.11.2017 and 3914(E) dated 08.12.2017 shall continue to be governed under para 19 as stated in the said notifications except the ceiling prices stated above.
- Consequent to the issue of ceiling prices of such formulations as specified in column (2) of the above table in this notification, the price order(s) fixing ceiling or retail price, if any, issued prior to the above said date of notification, stand(s)














 3. The New Registration page is displayed. Select the New Registration option.
3. The New Registration page is displayed. Select the New Registration option.
 15. Click the PROCEED button.
15. Click the PROCEED button.